Abstract
Context:
COVID-19 is known to cause extrapulmonary manifestations, including gastrointestinal and abnormal liver functions. Multiple mechanisms have been proposed to explain the pathobiology of liver damage: ACE2 receptor cholangiocytes mediated systemic inflammation, cytokine storm, hyperinflammation, and hypoxic changes. This was a cross-sectional study done in Department of General Medicine,JJM Medical College Davangere between July and September 2020 of patients falling under Category B and Category C.
Materials and Methods:
The aim is to describe the clinical characteristics in patients of COVID-19 and investigate the gender difference with particular regard to liver impairment.Confirmation of COVID-19 positivity was based on RT-PCR for SARS-CoV-2 RNA. Laboratory investigations and clinical data was analyzed using SPSS Statistics 27.
Results:
The final study population consisted of 116 patients. On performing the Mann Whitney U test, adjusted P values reveal a significant difference in ALT (P = 0.0348), total bilirubin (P = 0.0012) and direct bilirubin (P = 0.0024). The degree of hypoalbuminemia in males was significantly higher than in females (P = 0.0075). Other biochemical parameters, however, did not show significant difference amongst patients based on gender. Acute kidney injury was the most prevalent condition, present in 67.2% of the patients. Other co-morbidities were diabetes mellites, chronic liver disease, hypertension, hepatitis B and C, and hypothyroidism.
Conclusion:
Ultrasonography of the abdomen is an essential investigation for all patients testing positive for COVID-19. Pre-existing disease may aggravate the viral hepatic injury, thereby worsening the clinical outcome. The profiles of liver toxicity of the drugs used in the treatment of COVID-19 also warrant watchful monitoring of liver function.
Keywords: Coronavirus, hypoalbuminemia, liver function test
Introduction
In December 2019, a novel coronavirus was identified as the pathogen to cause pneumonia in China, temporarily named 2019-Novel Coronavirus (nCoV) by the World Health Organization.[1,2] On February 11, 2020, based on the phylogeny, taxonomy, and established practice, 2019-nCoV was officially named severe acute respiratory syndrome coronavirus (SARS)-CoV-2, and the disease caused by SARS-CoV-2 was named Coronavirus Disease (COVID-19).[3,4]
COVID-19 is usually asymptomatic in most cases, and when symptomatic, it causes a flu-like illness characterized by dry cough, sore throat, fever, dyspnea, myalgia, and other symptoms. It can be complicated by interstitial pneumonia with severe acute respiratory syndrome (SARS) and superimposed bacterial infections, which may be fatal. While COVID-19 is known to cause an extensive pulmonary disease, clinicians have observed many extrapulmonary manifestations of COVID-19, including gastrointestinal and abnormal liver functions.
Multiple mechanisms have been proposed to explain the pathobiology of liver damage. The SARS-CoV-2 virus may bind to the angiotensin-converting enzyme 2 (ACE2) on cholangiocytes, leading to cholangiocyte dysfunction, and induces a systemic inflammatory response culminating in liver injury.[5] Cytokine storm, a consequence of hyper inflammation and metabolic derangements induced by hypoxia, also leads to hepatocyte injury.[6] Liver injury in the setting of COVID-19-related illness poses a unique challenge to the physician. First, there is uncertainty whether there is a pre-existing undiagnosed liver disease. Second, many of the medications used to treat moderate and severe COVID-19 have their liver toxicity profiles. Finally, in the subset of patients who experience critical illnesses, multiple factors may influence the trajectory of the liver injury.[7]
Currently, limited data are available that link the underlying liver injury with the severe SARS-CoV-2 infection. Hence, this study aims to describe the clinical characteristics of COVID-19 and investigate the gender difference regarding liver impairment.
Materials and Methods
This study was a retrospective cross-sectional study between July and September 2020 of the patients admitted to the Chigateri General Hospital attached to JJM Medical College, Davangere. The cases were defined based on the interim guidelines issued by the Ministry of Health and Family Welfare, Government of India. The admitted patients with the respiratory rate exceeding 24 cycles per minute and oxygen saturation below 94% without oxygen supplementation (falling under Category B and Category C) were included in the study.[8] Patients on mechanical ventilation, tocilizumab, pirfenidone, azathioprine, and cyclosporine were excluded from the study. Patients on regular hepatotoxic medications were also excluded from the study. Confirmation of COVID-19-positivity was based on real-time polymerase chain reaction (RT-PCR) for SARS-CoV-2 Ribonucleic acid of nasopharyngeal and oropharyngeal swabs of patients.
The study began after obtaining clearance from the Institutional Ethics Committee. Detailed clinical history was noted, and a physical examination was performed. Laboratory investigations sent included complete hemogram, liver function tests (LFTs), i.e., alanine and aspartate aminotransferases, total, direct and indirect bilirubin levels, albumin, and lactate dehydrogenase. Chest X-ray, electrocardiography, and renal function tests were also ordered. [Table 1] presents the definitions of abnormal biochemical parameters.
Table 1.
Definition of abnormal biochemical parameters
| Biochemical Parameters | Values |
|---|---|
| Raised LDH | >280 IU/L |
| Transaminitis | AST OR ALT >80 IU/L |
| Hyperbilirubinemia | Total Bilirubin >1.5mg/dL |
| Acute Kidney Injury | Creatinine >1.2 mg/dL |
| Hypoalbuminemia | Albumin <3.5g/dL |
| Uremia | Urea >40 mg/dL |
The demographic data, comorbid conditions, and abnormal biochemical parameters were presented as descriptive statistics. The gender differences were analyzed using the Mann–Whitney U test for non-parametric data. For Gaussian distributions, the t-test was employed. Fisher’s exact analysis was performed on patients with deranged liver function. The data analysis was performed using the IBM SPSS Statistics 27. The demographic data, comorbid conditions, and abnormal biochemical parameters were presented as descriptive statistics in [Table 2.]
Table 2.
Descriptive statistics of biochemical parameters
| Biochemical Parameter | Mean | Standard Deviation | Median | Minimum | Maximum |
|---|---|---|---|---|---|
| Direct bilirubin (mg/dL) | 0.31 | 0.34 | 0.21 | 0.02 | 2.06 |
| Indirect bilirubin (mg/dL) | 0.39 | 0.29 | 0.3 | 0 | 1.64 |
| Total Bilirubin (Total) (mg/dL) | 0.7 | 0.59 | 0.52 | 0.12 | 3.28 |
| Serum Albumin (g/dL) | 3.46 | 0.73 | 3.45 | 0.25 | 5 |
| ALT (IU/L) | 94.47 | 399.5 | 31.7 | 6.6 | 4037.9 |
| AST (IU/L) | 73.27 | 83.34 | 43.9 | 19.5 | 672 |
| Lactase Dehydrogenase (mg/dL) | 791.33 | 490.25 | 614 | 105 | 2401 |
| Serum Creatinine (mg/dL) | 2.18 | 2.88 | 1.31 | 0.5 | 18.52 |
| Serum Urea (mg/dL) | 48.86 | 72.17 | 25.3 | 6.1 | 535.4 |
| C Reactive Protein (mg/L) | 61.95 | 55.56 | 56 | 0.6 | 292.7 |
Results
The final study population consisted of 116 patients out of which 72 were males, and 44 were females [Figure 1]. The mean age of the males being 48.2 ± 14.6 years, and females 50.1 ± 13.8 (range 17–80 years).
Figure 1.
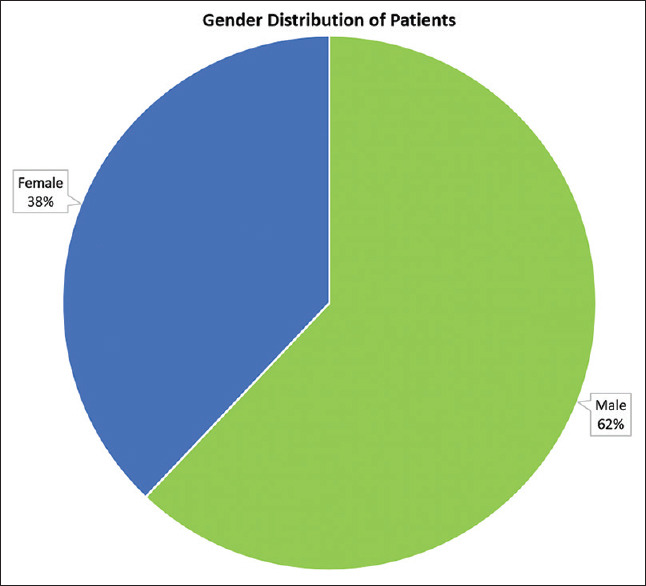
Gender distribution of patients
In the study population, 14 patients had no comorbidity (12.1%). The remaining patients had one or more of the following comorbidities: acute kidney injury, diabetes mellitus, chronic liver disease, hypertension, hepatitis B and C, and hypothyroidism [Figure 2]. Acute kidney injury was the most prevalent condition present in 67.2% of the patients.
Figure 2.
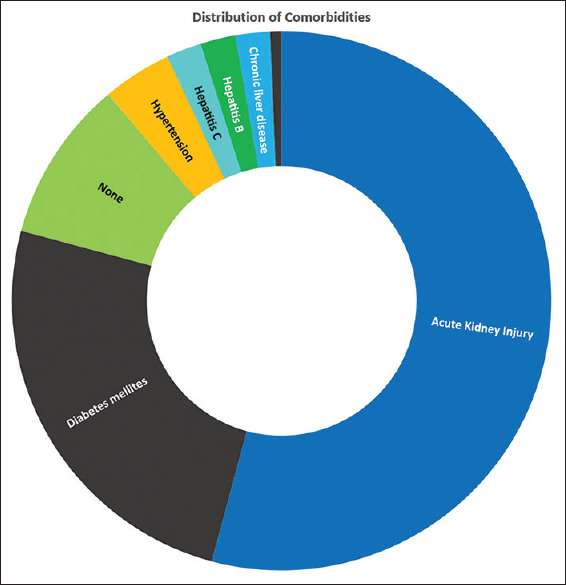
Distribution of comorbidities among the study population
In the study population, alanine transaminase (ALT) was elevated in 40 males (>33 IU/l) and 46 females (>25 IU/l); aspartate transaminase (AST) was elevated (>48 IU/l) in 35 males and 12 females. The total bilirubin was raised (>1.5 mg/dL) in five males and five females. Direct bilirubin was raised (>0.3 mg/dL) in 27 males and eight females, and indirect bilirubin in six males and three females. Fifty-one males and 27 females had elevated levels of serum creatinine (>1.2 mg/dL). The albumin levels were low (<3.5 g/dL) in 32 males and 31 females. [Table 3 and Figure 3] summarize the results.
Table 3.
Biochemical abnormalities—males and females
| Biochemical Parameters Deranged | Male | Female | ||
|---|---|---|---|---|
|
|
|
|||
| Number of Patients | Percentage of Patients | Number of Patients | Percentage of Patients | |
| Raised ALT (IU/L) | 40 | 55.6 | 36 | 81.8 |
| Raised AST (IU/L) | 35 | 48.6 | 12 | 27.3 |
| Direct Hyperbilirubinemia (mg/dL) | 27 | 37.5 | 8 | 18.2 |
| Indirect Hyperbilirubinemia (mg/dL) | 6 | 8.3 | 3 | 6.8 |
| Hyperbilirubinemia (Total) (mg/dL) | 9 | 12.5 | 5 | 11.4 |
| Raised Serum Creatinine (mg/dL) | 51 | 70.8 | 27 | 61.4 |
| Hyperalbuminemia (g/dL) | 32 | 44.4 | 31 | 70.5 |
| Raised Serum Urea (mg/dL) | 47 | 65.3 | 26 | 59.1 |
| Raised LDH (mg/dL) | 71 | 98.6 | 43 | 97.7 |
Figure 3.
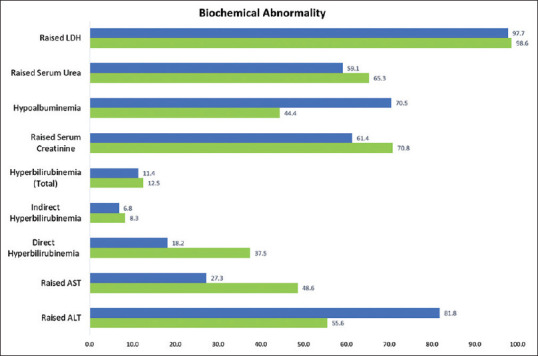
Abnormal biochemistry–males and females
Albumin, transaminases, and direct bilirubin show some variability among COVID-positive men and women. On performing the Mann-Whitney U test, adjusted P values reveal a significant difference in ALT (P = 0.0348), total bilirubin (P = 0.0012), and direct bilirubin (P = 0.0024) levels among the males and females.
Fischer’s exact analysis was performed on patients with deranged LFTs (as defined in Table 1). The degree of hypoalbuminemia in the males was significantly higher than in the females (P = 0.0075). Other biochemical parameters, however, did not show significant differences among the patients based on gender.
Discussion
Angiotensin converting enzyme (ACE2), the host cell receptor for SARS, has also been implicated in mediating the 2019-nCoV infection. It is postulated that the virus directly binds to ACE2 positive cholangiocytes, but not necessarily hepatocytes. This suggests a potential mechanism of damage to the bile ducts by the virus using ACE2 as host cell receptors.[9] The autopsy analysis of the liver tissues of the patients who died of SARS showed fatty degeneration and central lobular necrosis, substantiating the hypothesis that SARS-CoV-2 can also attack the human liver.[10] A direct viral cytopathic effect cannot be ruled out, given ACE2 receptors in the hepatocytes. The robust inflammatory response is characterized by very high levels of Interleukin-6, which has been implicated in both the inflammatory and the repair responses in liver disease.[7]
Acute kidney injury was the most commonly present comorbidity among patients (67.2%) with moderate to severe disease. This is explained by acute tubular necrosis, podocytopathy, microangiopathy, rhabdomyolysis, and collapsing glomerulopathy.[11] Viral infection increases tubular damage by activating the membrane attack complex. There is infiltration of CD68+ macrophages into the tubule-interstitium. Tubular necrosis, vacuolar degeneration, and loss of brush border are very common autopsy-associated findings. Also, diffuse erythrocyte aggregation with endothelial damage and obstruction without fibrin thrombi or distinct fragmentation of erythrocytes or platelets were observed after autopsies. Clusters of SARS-CoV-2 were found with electron microscopy in the tubular epithelium and podocytes. The cytokine storm increases apolipoprotein L1 gene expression leading to podocyte injury. Other indirect mechanisms that potentially lead to tubular injury are sepsis, cytokine storm syndrome, shock/hemodynamic instability, rhabdomyolysis, and hypoxia of kidney tissue. Renal biopsies are hard to perform given the respiratory and hemodynamic instability of AKI patients and the use of anticoagulation which increases the risk of bleeding.[12]
Transaminitis was the most common biochemical derangement of liver function: 65.5% of all the patients having raised ALT and 40.2% raised AST. An interesting gender difference was observed in the raised ALT levels among men and women. Although the correlation is significant, we propose to attribute this difference to the physiological liver metabolic variation between the two sexes.
Of all the patients with deranged LFTs, hypoalbuminemia was significantly more severe in men with COVID-19 in comparison with women. Pre-existing or underlying hepatic pathology may explain this difference. A possible confounder could be a higher prevalence of alcoholism among men. As an inverse acute phase reactant, lower albumin levels reflect severe inflammation and the cytokine storm, thus suggesting a poor prognosis in COVID-19.[13,14]
In the context of primary healthcare, liver dysfunction provides a forewarning of the impending severe disease. Early detection and prompt referral can play a crucial role in controlling morbidity and subsequent mortality in COVID-19.
Conclusion
Ultrasonography of the abdomen is an essential investigation for all patients testing positive for COVID-19. Pre-existing diseases may aggravate the viral hepatic injury, thereby, worsening the clinical outcome. The liver toxicity profiles of the drugs used in the treatment of COVID-19 also warrant watchful monitoring of the liver function.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–73. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus:Classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–44. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. WHO Director-General's remarks at the media briefing on 2019-nCoV on 11 February 2020. [Last accessed on 2021 Mar 03]. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020 .
- 5.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of 2019 novel coronavirus infection in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine storm in COVID-19:The current evidence and treatment strategies. Front Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaefer EAK, Arvind A, Bloom PP, Chung RT. Interelationship between coronavirus infection and liver disease. Clin Liver Dis (Hoboken) 2020;15:175–80. doi: 10.1002/cld.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical management protocol:COVID-19. Government of India Ministry of Health and Family Welfare Directorate General of Health Services (EMR Division) Version 5.03.07.20. [Last accessed on 2021 Mar 03]. Available from: https://www.mohfw.gov.in/pdf/ClinicalManagementProtocolforCOVID19.pdf .
- 9.Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. BioRxiv. 2020 doi:10.1101/2020.02.03.93176. [Google Scholar]
- 10.Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, et al. Clinical features of COVID-19-related liver functional abnormality. Clin Gastroenterol Hepatol. 2020;18:1561–6. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batlle D, Soler MJ, Sparks MA, Hiremath S, South AM, Welling PA, et al. Acute kidney injury in COVID-19:Emerging evidence of a distinct pathophysiology. J Am Soc Nephrol. 2020;31:1380–3. doi: 10.1681/ASN.2020040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benedetti C, Waldman M, Zaza G, Riella LV, Cravedi P. COVID-19 and the Kidneys:An update. Front Med (Lausanne) 2020;7:423. doi: 10.3389/fmed.2020.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Violi F, Cangemi R, Romiti GF, Ceccarelli G, Oliva A, Alessandri F, et al. Is albumin predictor of mortality in COVID-19. Antioxid Redox Signal. 2021;35:139–42. doi: 10.1089/ars.2020.8142. [DOI] [PubMed] [Google Scholar]
- 14.Aziz M, Fatima R, Lee-Smith W, Assaly R. The association of low serum albumin level with severe COVID-19:A systematic review and meta-analysis. Crit Care. 2020;24:255. doi: 10.1186/s13054-020-02995-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


