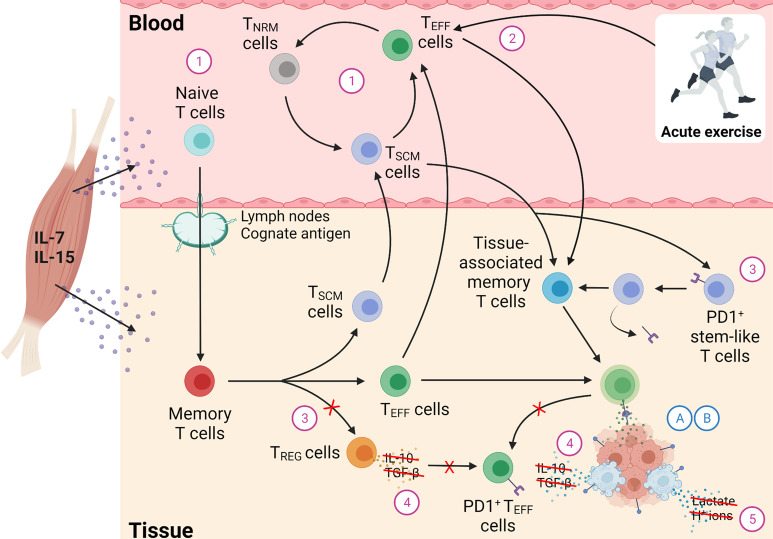
Figure 2.
A synthesis of possible candidate mechanisms explaining how T cell regulation is augmented by physical activity leading to enhanced T cell function against tumour cells, culminating in the preservation of cancers in the equilibrium phase of the immunoediting process. In this hypothetical process, we illustrate how regular exercise might alter features of T cell success contained within the cancer immunogram, which in turn may explain how regular exercise maintains immune control of immunogenic tumours. Each candidate component is driven by the effects of immunoregulatory cytokines (e.g., IL-7, IL-15), secreted from skeletal muscle in people who are physically active, on immune competency. The candidate components included in this model are: (1) Physical activity may alter general T cell status in blood by preserving naïve T cell frequency in ageing, thus enabling T cells to identify a greater diversity of tumour antigens in older age. As outlined in Determinant 1 however, we note that there is not conclusive evidence yet that naïve T cell output is maintained by physical activity in ageing. Instead, following antigen encounter, regular exercise may revert antigen-experienced memory T cells and preserve these as stem cell-like cells such as TNRM cells and TSCM cells, with a surface phenotype similar to naïve T cells. This maintenance of stem cell-like T cells (such as TSCM and TNRM cells) may augment the supplementation of tissue-associated memory T cells, thus leading to more persistent anti-tumour responses when required. (2) Acute exercise may augment the redistribution and infiltration of CD8+ TEFF cells, as well as other T cell subsets, to tissue sites harbouring tumour antigens. However, as outlined in Determinant 2, we note that there are incompatibilities in the theory that exercise acutely mobilises effector cells to sites harbouring tumours. (3) Regular exercise may alter immune checkpoint expression, by preferentially suppressing development of CD4+ TREG cells in tissue sites harbouring tumour antigens, averting T cell anergy and leading to more robust effector responses against cancer cells. In addition, regular exercise may reverse exhaustion to PD1+ stem cell-like CD8+ T cells – upon chronic exposure to antigen and immunosuppressive cytokines – which may help supplement the frequency of tissue-associated memory T cells with capacity to elicit persistent effector responses. (4) In tandem to Determinant 3, regular exercise may alter the presence of inflammatory mediators within the tumour microenvironment – namely IL-10 and TGF-β – by suppressing the development of TREG vs. TEFF, thus alleviating immunosuppressive signalling within the tumour microenviornment to enhance T cell killing of cancer cells. (5) Exercise training may alter metabolic inhibitors of T cell activity within the tumour microenvironment, via the enhancements to immune competency discussed in Determinants 1-3, which increase T cell killing of cancer cells to reduce lactate accumulation, acidosis, and hypoxia which arise from the tumour cells themselves. Importantly, each component in this model is dependent on tumour-intrinsic factors: (A) immunogenicity (i.e., tumour ‘foreignness’), and (B) MHC-1 expression (i.e., ‘visibility’), and therefore the anti-cancer benefits of physical activity are unlikely to be seen in the absence of tumour immunogenicity and MHC-1 expression. TREG cells, Regulatory T cells; TEFF cells, Effector T cells; TSCM cells, Stem-like T cells; TNRM cells, Naïve-revertant memory T cells. Created with BioRender.com.