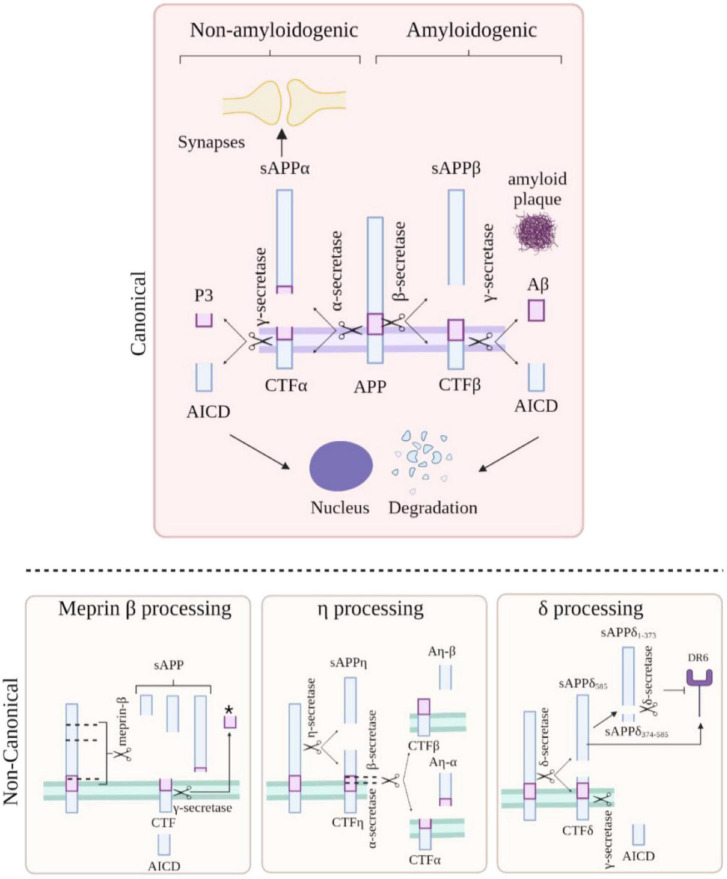
FIGURE 2.
Amyloid precursor protein (APP) cleavage. APP can undergo canonical (top) and non-canonical (bottom) processing. In the amyloidogenic pathway (top, right side), APP is processed by β-secretase and γ-secretase resulting in the formation of Aβ peptides, APP intracellular domain (AICD) and sAPPβ. In the non-amyloidogenic pathway (top, left side), APP is cleaved by α-secretase and γ-secretase resulting p3 peptide, AICD, and sAPPα. Meprin-β cleavage (bottom left) generates three soluble APP fragments; the remaining CTF fragment can be further cleaved by γ-secretase giving rise to a smaller fragment indicated by * and AICD. APP cleavage by η-secretase (bottom, middle panel) generates an APP (sAPPη) and a CTFη fragment which can be further processed by either α or β-secretase and then by γ-secretase resulting in the formation of Aη-α/β and a CTFα or β fragment. APP cleavage by δ-secretase (bottom right) gives rise to a fragment which can activate the death cell receptor (DR6) promoting cell death or can be further cleaved to generate a fragment unable to bind the receptor; the remaining CTFδ fragment can be processed by γ-secretase form the intracellular domain AICD. Created with BioRender.com.