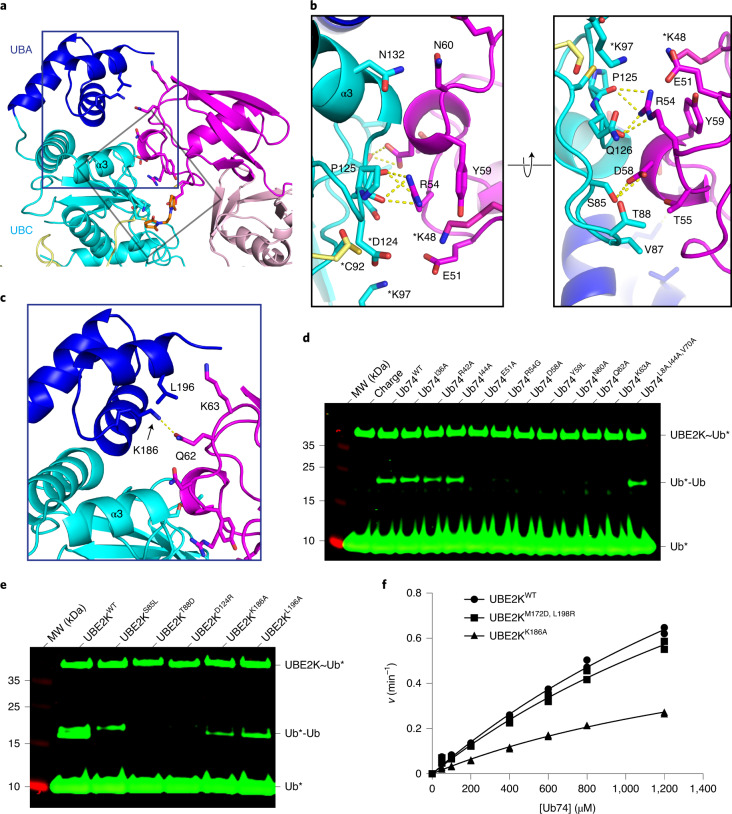
Fig. 3. UBE2K acceptor site employs UBC and UBA to orient UbA.
a, UbA (magenta) is encased by UBE2K UBC (cyan) and UBA (blue). b, Close-up views of key residues (sticks) between UbA and UBC domain in a. Hydrogen bonds are shown as dotted lines. Asterisks (*) indicate modeled residues. c, Interaction between UbA and UBA domain with key residues shown as sticks. d, Nonreduced SDS–PAGE showing UBE2K-catalyzed di-Ub formation with UbA variants. I36, R42 and I44 are remote from the UBE2K/UbA binding interface. e, Nonreduced SDS–PAGE showing di-Ub formation using UBE2K with acceptor-site mutations. Asterisks (*) in d and e indicate fluorescent-labeled UbD. f, Kinetics of di-Ub formation catalyzed by UBE2K variants. Data from two independent experiments (n = 2) were fitted with the Michaelis–Menten equation. kcat/Km values (UBE2KWT = 750 M−1 min−1, UBE2KM172D,L198R = 684 M−1 min−1 and UBE2KL186A = 355 M−1 min−1) were estimated from the slope of the linear portion of curve.