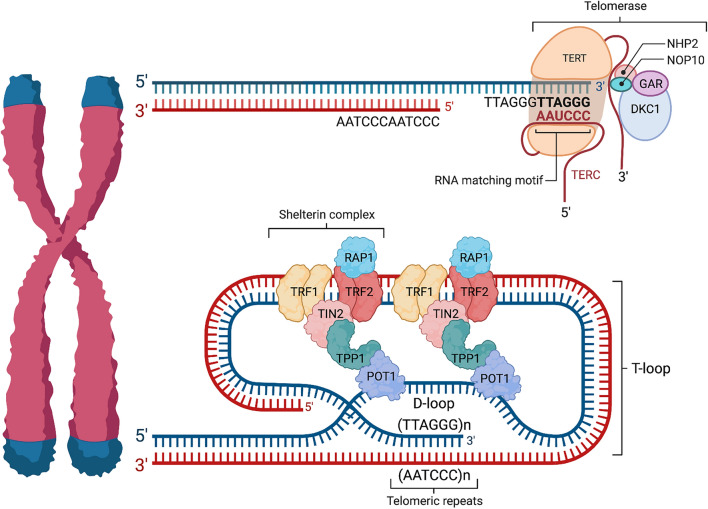
Fig. 1.
Telomere structure, shelterin complex and telomerase. Telomeres are composed of long arrays of tandem TTAGGG repeats that are heterogenous in length and can reach up to 15 kb. The G-rich lagging strand ends in a 3′ single-stranded overhang of about 50–200 nucleotides. The overhang folds back and pairs with the C strand in the double-stranded DNA, which form a D-loop that results in a higher-order DNA structure called the t-loop. The TTAGGG/CCCTAA duplexes are bound by TRF1 and TRF2, two members of the shelterin complex. The subunit TIN2 (TRF1-interacting nuclear factor 2) bridges TRF1 and TRF2 while simultaneously interacting with a third partner, the subunit TPP1 (adrenocortical dysplasia protein homolog, also known as ACD). TPP1 interacts with POT1 (protection of telomere 1), which binds the 3′ single-stranded overhang via its two OB fold domains and consolidates the nucleoprotein complex cohesion. Finally, RAP1 (TRF2-interacting protein), the Shelterin’s last subunit, binds exclusively to TRF2. Telomerase is a retro-transcriptase whose holoenzyme is composed of a telomerase reverse transcriptase (hTERT) and an RNA component (hTERC or hTR) as well as several other protein factors, such as dyskerin (DKC1), NHP2, NOP10, and GAR1, which are also part of the H/ACA ribonucleoprotein complex. hTERC contains a sequence complementary to the telomeric DNA and serves as a matrix for telomere elongation