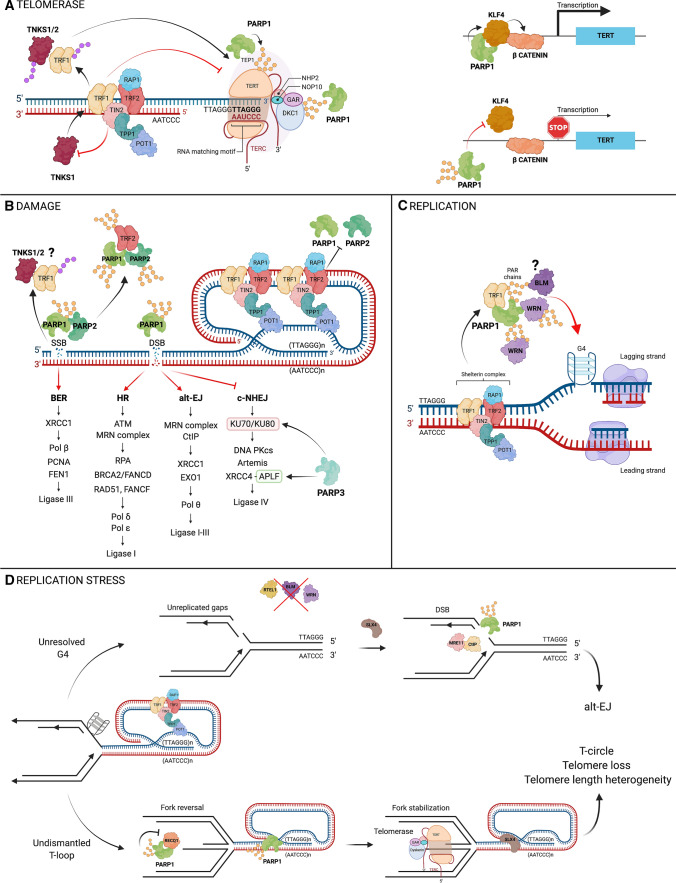
Fig. 3.
Schematics of the different telomere maintenance pathways involving ART enzymes. A Tankyrase-dependent PARylation of TRF1 displaces the protein from the DNA, which allows telomerase access to telomeres. TIN2 negatively regulates tankyrase 1. PARP1 can target protein components of the holoenzyme and/or control hTERT gene expression through PARP activity-independent regulation of KLF4. B Tankyrase activity is activated upon oxidative stress at telomeres and PARylates TRF1. PARP1 and PARP2 are recruited at telomeres upon oxidative stress and can PARylate TRF2 in vitro. Internal telomeric DSBs are repaired via PARP1-dependent alt-EJ. C During replication, telomeric G4s are unwound by the RECQ helicases WRN and BLM. PARP1 is activated upon treatment of cells by G4 ligands, which trigger the PARylation of TRF1 and recruitment of WRN and BLM. PARP1 is also able to PARylate WRN directly while WRN binds PAR in vitro. D Telomeres are highly sensitive to replication stress due to their t-loop structure and the formation of G4s. Preventing G4 unwinding by BLM leads to DSBs, which are repaired by PARP1-dependent alt-EJ. Deficiency in helicase RTEL1, as observed in several telomeropathies, leads to telomeric dysfunction due to an un-resolved t-loop. Stalling of the replication fork triggers fork reversal, whose chicken foot structure is recognized by telomerase. PARP1 prevents fork restart by PARylating RECQL1, which leads to telomeric dysfunction