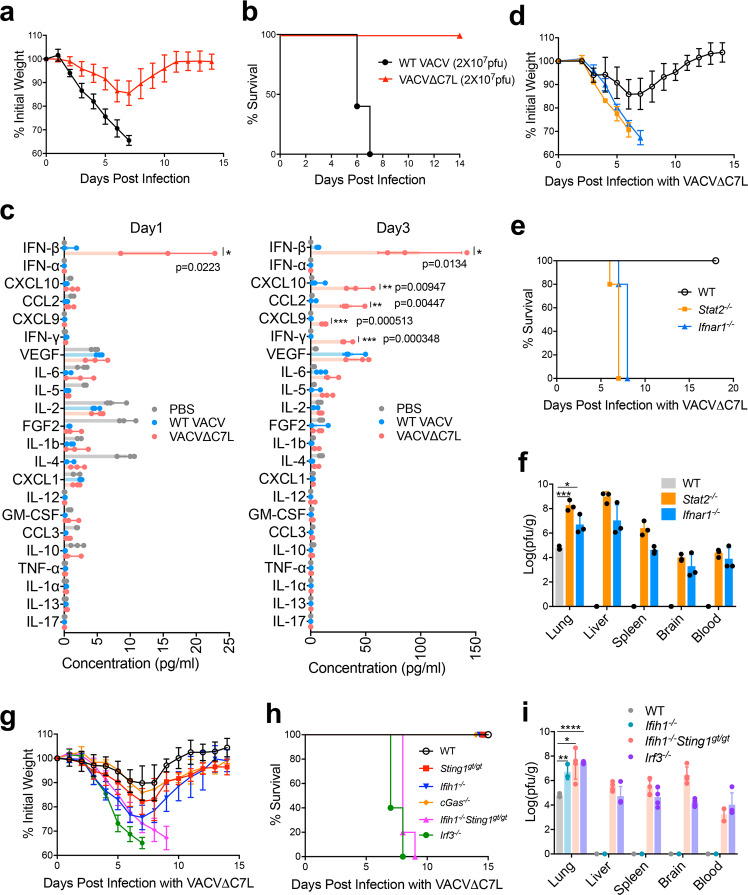
Fig. 1. Type I IFN signaling is essential for restricting replication and dissemination of vaccinia virus and C7 protein is a virulence factor for lethal infection.
a, b shown are the percentages of initial weight a or Kaplan–Meier survival curve b of WT C57BL/6J control mice (n = 5 in each group) over days post intranasal infection with WT VACV or VACV∆C7L at a dose of 2 × 107 pfu. c Levels of IFN-β and other cytokines/chemokines in BAL from VACV∆C7L or WT VACV (2 × 107 pfu)-infected mice collected at day1 and day 3 post infection determined by ELISA or Luminex. d, e shown are percentages of initial weight d or Kaplan-Meier survival curve e over days post intranasal infection with VACV∆C7L at a dose of 2 × 107 pfu in Stat2−/−, Ifnar1−/−, or age-matched WT C57BL/6J control mice (n = 5 in each group). f Titers of VACV∆C7L in the lungs, livers, spleens, brains and blood of Stat2−/−, Ifnar1−/−, or age-matched WT C57BL/6J control mice at day 4 post intranasal infection with VACV∆C7L at a dose of 2 × 107 pfu. Data are represented as mean ± SD (n = 3–5). *p = 0.0148, ***p = 0.000203. g, h shown are the percentages of initial weight g or Kaplan–Meier survival curve h over days post intranasal infection with VACV∆C7L at a dose of 2 × 107 pfu in cGas−/−, Stinggt/gt, Ifih1−/−, Ifih1−/−Sting1gt/gt, Irf3−/−, or age-matched WT C57BL/6J control mice (n = 5 in each group). i Titers of VACV∆C7L in the lungs, livers, spleens, brain and blood of Ifih1−/−, Ifih1−/−Sting1gt/gt, Irf3−/−, or age-matched WT C57BL/6J control mice at day 4 post intranasal infection with VACV∆C7L at a dose of 2 × 107 pfu. *p = 0.0166, **p = 0.0056, ****p = 0.0000008. Data are presented as mean ± SD (n = 3-5). *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001. Two-tailed unpaired Student’s t test was used for comparisons of two groups in the studies. Data are representative of two (c, f, i), or three (a, b, d, e and g, h) independent experiments. Source data are provided as a Source Data file.