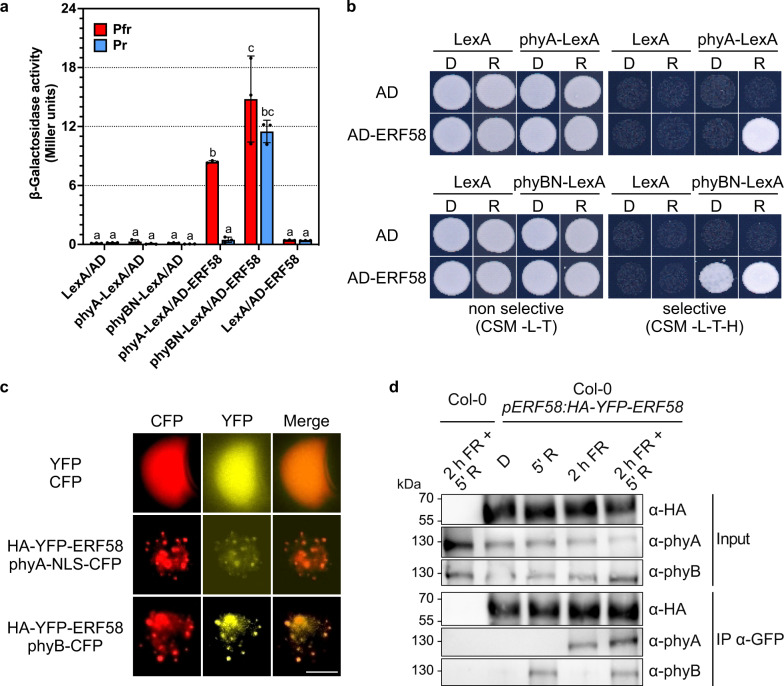
Fig. 1. ERF58 interacts with phyA and phyB.
a Y2H protein-protein interaction assay. Full-length phyA or the N-terminal half of phyB (phyBN) fused to LexA and ERF58 fused to the GAL4 AD were expressed in yeast. Yeast cells were grown in chromophore (PCB)-supplemented medium, exposed to R or FR light for 5 min to convert phytochromes to Pfr or Pr, and incubated in the dark for another 4 h. β-Gal activity was then measured using an ONPG assay. Bars show mean β-Gal activity of three replicates ±SD. Different letters indicate significant differences as determined by two-way ANOVA followed by post-hoc Tukey’s HSD test; p < 0.05. b Y2H growth assay. phyA- or phyBN-LexA and AD-ERF58 were expressed in yeast. Yeast cells were grown on CSM -L-T plates or CSM -L-T-H plates supplemented with PCB. Plates were incubated in the dark (D) or R light (R). c Co-localisation of ERF58 with phyA and phyB in tobacco. p35S:HA-YFP-ERF58 and either p35S:PHYA-NLS-CFP or p35S:PHYB-CFP were transiently co-expressed in tobacco leaf epidermis cells by agro infiltration; empty p35S:YFP and p35S:CFP vectors were used as control. YFP and CFP signals were detected by epifluorescence microscopy. Scale bar represents 5 μm. d Co-immunoprecipitation of phyA and phyB with ERF58. Stable transgenic Arabidopsis lines expressing pERF58:HA-YFP-ERF58 in Col-0 background were used for Co-IP; Col-0 was used as negative control. Three day old dark-grown seedlings were treated for 5 min with R light, for 2 h with FR light, for 2 h with FR light followed by 5 min R light, or kept in the dark and used for Co-IP. Eluate fractions were analysed by SDS-PAGE and immunoblotting with α-phyA, α-phyB, or α-HA antibodies. Experiments in b–d were repeated three times with similar results.