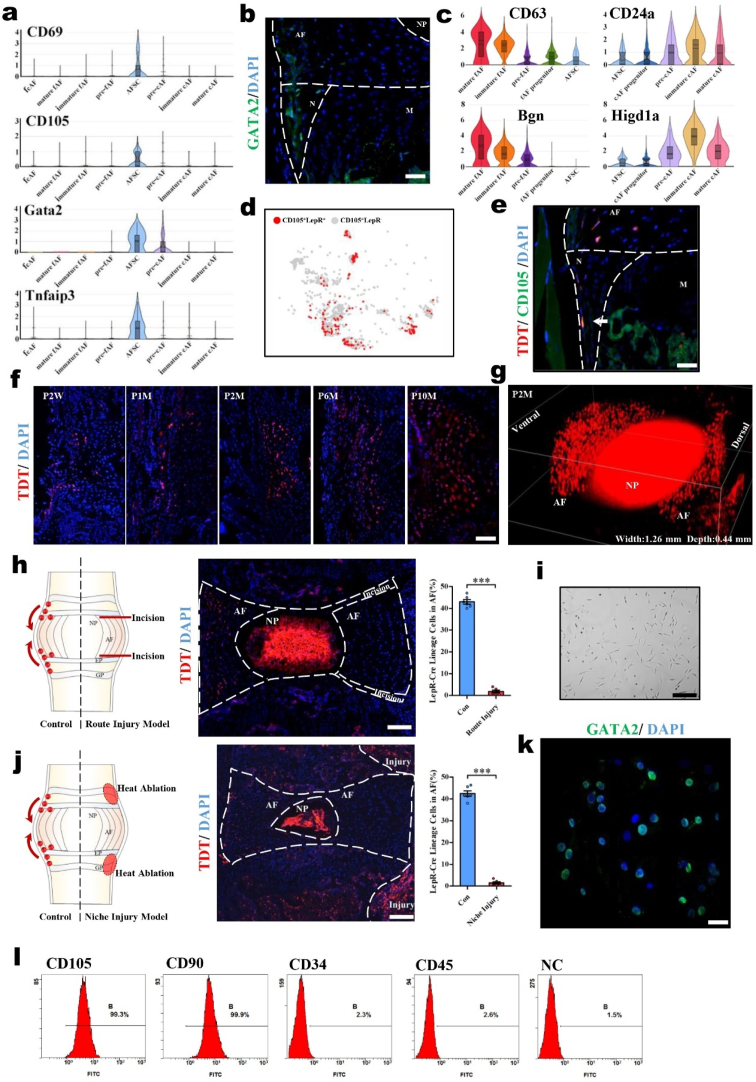
Fig. 2.
AFSCs are appropriate seed cells for AF reconstruction with the abilities to migrate and differentiate. (a) Violin plots showing the expression levels of stem cell markers for AF populations. (b) Immunofluorescence staining of GATA2 in mouse IVDs (P2M). (c) Violin plots showing the expression levels of differentiation-related genes in AF populations. (d) Dot plots showing the LepR+ cells in the CD105+ cells on the t-SNE map. (e) Immunofluorescence staining of CD105 in the IVDs of LepR-cre; tdTomato mice (P2M). Scale bar, 50 μm. Arrowhead, colocalization of CD105 and tdTomato. M, metaphysis; N, niche. (f) Immunofluorescence images of LepR-cre; tdTomato mice of different ages. Scale bar, 100 μm. P, postnatal. W, weeks. M, months. (g) 3D reconstruction of the immunofluorescence images of lumbar IVDs in LepR-cre; tdTomato mice (P2M). Schematic diagrams, immunofluorescence images, and the tdTomato+ cells statistical data of (h) the migratory route injury model and (j) the niche injury model of LepR-cre; tdTomato mice. Scale bar, 100 μm. (i) Cell morphology of cultured AFSCs. Scale bar, 50 μm. (k) Immunofluorescence staining of GATA2 in cultured AFSCs. Scale bar, 20 μm. (l) Flow cytometric analysis of CD105, CD90, CD34, and CD45 expression in cultured AFSCs (n = 6, ***P < 0.001 vs. control group. For all the above-mentioned statistical analyses, significance was determined by student's-t tests and the results were shown as mean ± SD.).