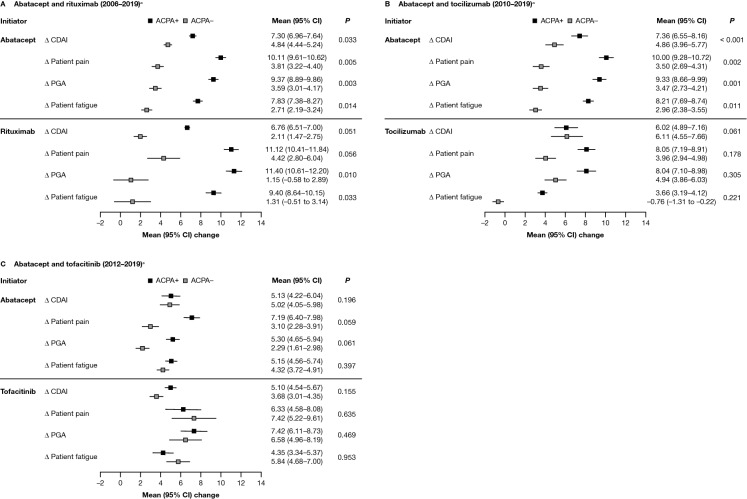
Fig. 2.
Adjusted mean improvement from baseline in disease and disability outcomes at 6 months after index date, by ACPA status, for patients who initiated abatacept or another non-TNFi b/tsDMARD.* A Abatacept and rituximab (2006–2019)a. B Abatacept and tocilizumab (2010–2019)a. C Abatacept and tofacitinib (2012–2019)a. *Adjusted for baseline covariates that differed by ACPA status (P < 0.1), not including factors that reduced the sample size by > 10% or were correlated with CDAI. Only the main variable category is listed below, although some variables were further broken down within each category: Adjusted variables for the 2006–2019 cohort included: for both drugs – BMI, marital status, smoking status, and prednisone use; for abatacept only – sex, race/ethnicity, insurance, college, work status, duration of RA, ACR functional class, history of malignancies, history of hypertension, history of serious infection, and current combination therapy; and for rituximab only – history of COPD. Adjusted variables for the 2010–2019 cohort included: for both drugs – race/ethnicity, insurance, work status, duration of RA, ACR functional class, and CDAI; for abatacept only – sex, marital status, smoking status, history of malignancies, history of hypertension, history of serious infections, current combination therapy, morning stiffness, and initiation year; and for tocilizumab only – age, history of CVD, and prednisone use. Adjusted variables for the 2012–2019 cohort included: for both drugs – college, duration of RA, and CDAI; for abatacept only – race/ethnicity, BMI, marital status, work status, and initiation year; for tofacitinib only – history of CVD, history of serious infections, prior non-TNFi use, current combination therapy, and patient pain. aTime period of initiation; refer to the Methods section for full details. Δ change, ACR American College of Rheumatology, ACPA+ anti-citrullinated protein antibody positive (anti-CCP2 ≥ 20 U/ml), ACPA− anti-citrullinated protein antibody negative (anti-CCP2 < 20 U/ml), anti-CCP2 anti-cyclic citrullinated peptide-2, b/tsDMARD biologic or targeted-synthetic disease-modifying antirheumatic drug, BMI body mass index, CDAI Clinical Disease Activity Index, CI confidence interval, COPD chronic obstructive pulmonary disease, csDMARD conventional-synthetic disease-modifying antirheumatic drug, CVD cardiovascular disease, PGA patient global assessment, RA rheumatoid arthritis, TNFi tumor necrosis factor inhibitor