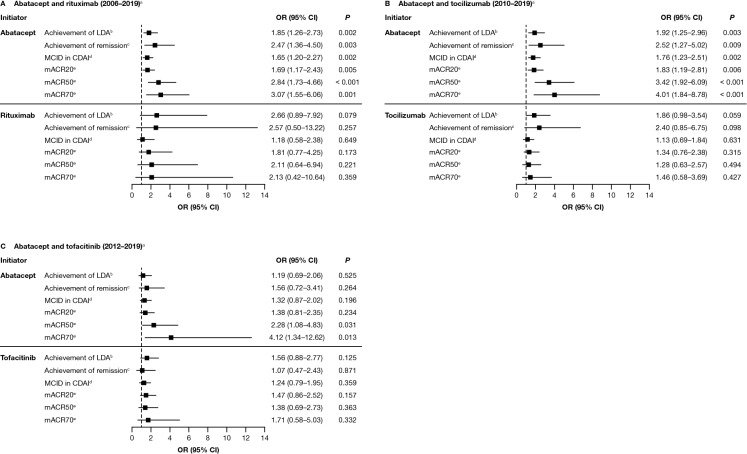
Fig. 3.
Adjusted association between ACPA status and achieving a clinical response to treatment with abatacept or another non-TNFi b/tsDMARD at 6 months after index date.* A Abatacept and rituximab (2006–2019)a. B Abatacept and tocilizumab (2010–2019)a. C Abatacept and tofacitinib (2012–2019)a. Data are presented as odds ratio (95% CI). *Adjusted for baseline covariates that differed by baseline CCP status (P < 0.1), not including factors that reduced the sample size by > 10% or were correlated with CDAI: adjusted variables for each cohort are listed in the footnote of Fig. 2. aTime period of initiation; refer to the Methods section for full details. bCDAI ≤ 10 (among those with moderate or higher disease activity). cCDAI ≤ 2.8 (among those with LDA or higher). dDrop of > 1 if LDA, drop of > 6 if moderate disease activity, and drop of > 12 if severe disease activity. emACR criteria based on two out of four measures (not using ESR or CRP). ACPA anti-citrullinated protein antibody, CCP cyclic citrullinated peptide, CDAI Clinical Disease Activity Index, CI confidence interval, CRP C-reactive protein, ESR erythrocyte sedimentation rate, LDA low disease activity, mACR20/50/70 20/50/70% improvement in modified American College of Rheumatology criteria, MCID minimal clinically important difference, OR odds ratio, TNFi tumor necrosis factor inhibitor. Figure adapted from Harrold LR, et al. ACR/ARP Annual Meeting; November 8–13, 2019; abstract number: 1386. Reprinted from ACR Convergence held November 8–13, 2019. The American College of Rheumatology does not guarantee, warrant, or endorse any commercial products or services. Reprinted by Springer