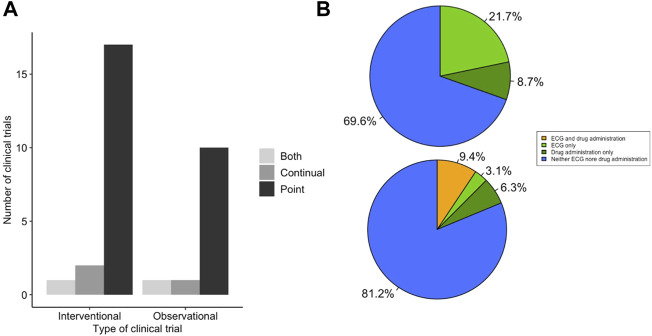
FIGURE 5.
QT-prolongation drug trials integrate drug and ECG timing to a low level. (A) Method of ECG recording for QT prolongation drug clinical trials. Trials were scored for whether outcomes reported ECG measurements determined by point ECG measurement (black), continual using Holter monitor (dark grey) or outcomes that used both point and continuous measurements (light grey). Interventional trials are classified as those involving some form of pharmaceutical challenge. (B) Integration of both drug and ECG timing in clinical trials for LQTS (top) or QT-prolonging drugs (bottom). Trials that take into account timing information are highlighted for: both ECG and drug delivery (orange), only ECG measurement/analysis (light green) and only drug administration (dark green).