Figure 3.
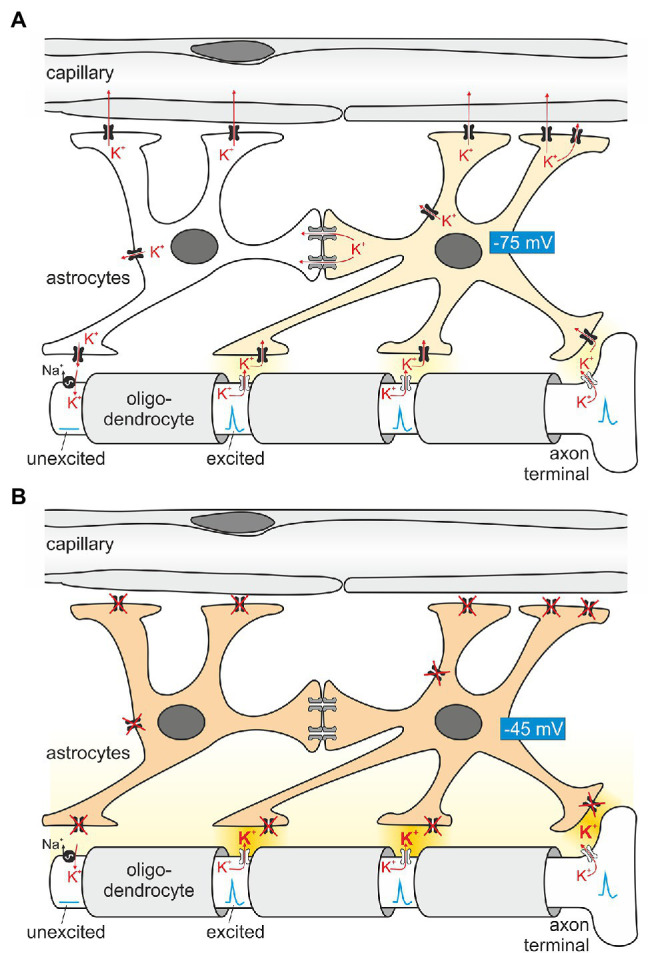
Principles of astroglial K+ buffering. (A) Astrocytes are in close contact with Ranvier nodes, exposed parts of the axon between segments wrapped with myelin (light gray) provided by oligodendrocytes. The nodes “regenerate” the action potential as it travels in a saltatory (jumping) mode to the next node. Conduction between nodes is electrotonic. When firing, Na+ enters the axon, depolarizes the nodal axolemma and K+ is leaving the axon through K+ channels (Kanda et al., 2019). The ensuing K+ exit from the axon increases extracellular K+ locally and induces K+ uptake by astrocyte processes in close proximity. A prerequisite of locally restricted K+ uptake by astrocytes is their hyperpolarized membrane voltage (requiring Kir4.1 channels depicted in black). On the other hand, K+ uptake depolarizes the astrocyte membrane and enhances the driving force for K+ exit at areas closer to blood vessels, where the K+ concentration is low. In addition, K+ is also transferred by electrical coupling to neighboring astrocytes which are, at this moment in time, not surrounded by increased K+. Once the nodal axon repolarizes, the axolemmal Na+/K+-ATPase takes up K+ released slowly by the astrocyte. (B) When astrocytes lack functional Kir4.1 channels, extracellular K+ is expected to rise (darker yellow extracellular space), and spontaneous action potential generation ensues, explaining the epilepsy seen in EAST patients.
