Abstract
Immunostimulants play an important role in the treatment of immunodeficiency. Macrophages are the first line in our immune defense system and play a critical role in the immune response. Therefore, finding new and better substances to induce an immune response by activating macrophages is an attractive research topic, especially in the fields of immunopharmacology and cancer prevention. Keratinocytes actively crosstalk with immune cells during wound repair, so enhancing the function of keratinocytes is also an important part of improving immunity. Beta-glucans are naturally occurring polysaccharides, consisting of d-glucose monomers linked by beta-glycosidic bonds. Several studies have investigated the immunomodulatory effects of beta-glucan, such as its anti-inflammatory and antibacterial properties. However, the use of yeast cell wall glucan has been limited because it is not soluble in water. In this study, we produced low-molecular-weight water-soluble yeast glucan (WSY glucan) and confirmed various aspects of its immune-enhancing effect. The structure of the beta-(1→3) and (1→6) bonds of WSY glucan were confirmed by nuclear magnetic resonance spectroscopy (1H-NMR) analysis. Our results showed that treatment with WSY glucan significantly and dose-dependently induced the production of inflammatory mediators (prostaglandin E2 (PGE2) and nitric oxide (NO)) and pro-inflammatory cytokines (tumor necrosis factor (TNF)-α and interleukin (IL)-6) in macrophages. In addition, WSY glucan treatment showed changes in the morphological structure of the macrophages and promoted phagocytic activity of the macrophages and wound healing in keratinocytes. Based on these results, WSY glucan is considered as a potential candidate for the treatment of diseases related to the weakening of the immune system without the limitation of insolubility.
Keywords: Water-soluble yeast beta-glucan, Immune activation, Macrophage differentiation, Phagocytosis, Immune mediators
Highlights
-
•
Soluble low-molecular-weight beta-glucan, WSY-glucan, was produced through enzymatic hydrolysis.
-
•
WSY glucan significantly and dose-dependently induced the production of pro-inflammatory cytokines (TNF-α and IL-6) and inflammatory mediators (NO and prostaglandin E2) in macrophages.
-
•
WSY glucan treatment showed changes in the morphological structure of the macrophages and promoted phagocytic activity of the macrophages and wound healing in keratinocytes.
1. Introduction
Immune stimulants activate innate immunity and promote the release of endogenous immune mediators as an important strategy in the treatment of immunodeficiency, chronic infection and cancer [1]. The dysregulated immune response in the elderly is mainly due to a weakened immune response, leading to an increased risk of infection and related complications in the elderly, therefore enhancing the immune response is very important for its improvement [2]. In cancer patients, activated immune cells such as macrophages and natural killer (NK) cells play a crucial role in the selective elimination of tumor cells [3]. Macrophages are the first line in our immune defense system and are effector cells of the innate immune system, which engulf bacteria and produce pro-inflammatory and antimicrobial mediators [1,4]. Macrophages also play an important role in clearing damaged cells through the process of programmed cell death. They phagocytose foreign substances such as pathogens, cancer cells, and dying cells, secrete a variety of cytokines such as interleukin-1β (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and cytotoxic molecules such as nitric oxide (NO) and reactive oxygen species (ROS) to regulate innate immunity and adaptive immunity [4,5]. Therefore, finding new and better substances to induce an immune response by activating macrophages is an attractive research topic, especially in the fields of immunopharmacology and cancer prevention [6]. As the main cell type in the skin, keratinocytes not only serve as structural cells, but also have important immune functions in defending the skin from infection [7]. Keratinocytes actively crosstalk with immune cells during wound repair, which is mediated by secreted signaling proteins such as cytokines and chemokines or through direct interactions [7,8]. When skin is injured, the migration and proliferation of keratinocytes at the wound edge occurs to initiate re-epithelialization [8]. Therefore, enhancing the function of keratinocytes is also an important part of improving immunity [7,8].
Beta-glucans are naturally occurring polysaccharides, consisting of d-glucose monomers connected by beta-glycosidic bonds [9]. They are essential structural elements of the cell wall and store energy in bacteria, and fungi (including yeast, algae, and plants), but do not exist in invertebrate and vertebrate tissue [10]. Knowledge about the health benefits of beta-glucan and its mechanism of action and relationship between structure and function continues to be studied [11], but many publications investigating the immunomodulating effects of beta-glucans such as immune-boosting, anti-inflammatory, and antimicrobial effects have been reported [10]. However, not all beta-glucans have immune-regulating functions. The primary chemical structure of the beta-glucan determines these properties. For example, cellulose, a type of (1,4)-beta-linked glucan, dose not exert immunomodulatory effects. On the contrary, yeast and fungi-derived beta-glucans, which are composed of (1,3)-beta-linked backbone with a small number of (1,6)-beta-linked side chains, are well known for their immunomodulating activities [12]. The difference of linkage and branching lead to various type of beta-glucan, which contribute to the tertiary structure, polymer charge, solubility, and the solution form (triple or single helix or random coil). Generally, insoluble beta-glucan from the yeast cell wall can be utilized as a functional food based on its excellent immune-enhancing effect [13,14]. However, cell wall beta-glucan is robust and difficult to solubilize, so there are many obstacles in absorption and delivery when it is used as a medicinal or cosmetic material. In this respect, improving the water solubility of yeast beta-glucan has been the subject of interest. The immune-enhancing effect of insoluble glucan has been widely studied, but the question of whether soluble glucan is also active in affecting the immune system needs to be confirmed in various experiments. In this study, we generated hydrolyzed water-soluble yeast glucan (WSY glucan) based on biotransformation using enzymes. In addition, efficacy experiments were performed to confirm the immunomodulatory efficacy of WSY glucan produced in this study. The results showed that treatment with WSY glucan significantly induced the activation of immune response and promoted keratinocytes migration. Based on our results, WSY glucan demonstrated potential as a candidate for the treatment of diseases related to a weakened immune system without the limitation of insolubility.
2. Materials and methods
2.1. Materials
Yeast beta-glucan was purchased from FocusHerb LLC (Xi'an, China). Depol 667p was purchased from Biocatalysts Ltd. (Parc Nantgarw, UK). Maltotriose (95%, MW 504.44 g/mol), Pullulan 1300 (for GPC, MW ∼1,300 g/mol), and Pullulan 6000 (for GPC, MW ∼5,900 g/mol) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine serum (FBS) and Dulbecco's Modified Eagle Medium (DMEM) were purchased from Gibco (Grand Island, NY, USA) and penicillin/streptomycin antibiotics were obtained from Invitrogen (Carlsbad, CA, USA). Water-soluble tetrazolium salt (WST-1 solution) was purchased from DoGenBio (Seoul, South Korea). Griess reagent, lipopolysaccharide (LPS), and TGF β1 were purchased from Sigma-Aldrich. The enzyme-linked immunosorbent assay (ELISA) kits for PGE2, TNF-α, and IL-6 were purchased from R&D Systems, Inc. (Minneapolis, MN, USA).
2.2. Preparation of water-soluble glucan
The hydrolyzation of yeast beta-glucan for the water-soluble form was carried out based on biotransformation using an enzyme. Five grams of yeast beta-glucan were mixed with 100 mL of water using an agitator for 1 h, and the suspension was pretreated at 100 °C. After cooling, the yeast beta-glucan was reacted with Depol 667p (2% w/w dry matter) to hydrolyze beta glucan for 3 h at 55 °C (pH range is 5.0–7.0). The reaction mixture was centrifuged at 8,000 rpm and 4 °C for 10 min. Subsequently, the supernatant was filtered by decompression with a filter paper having a pore size of 1 μm, and lyophilized and dissolved in water for this study.
2.3. High-performance size-exclusion chromatography
High-performance size-exclusion chromatography (HPSEC) was performed on a liquid chromatography system composed of a Waters 2695 Separation Module and a Waters 2414 Refractive Index Detector. Separation was conducted on a Yarra SEC-2000 column (Phenomenex, Torrance, CA, USA) with an isocratic mobile phase of water with a flow rate of 1 mL/min at 30 °C. Maltotriose, and pullulan 1,300 and 6,000 standards were used as reference molecules.
2.4. Nuclear magnetic resonance (NMR) spectroscopy
All NMR spectra were recorded on 500 MHz Bruker ADVANCE II NMR machine at the National Center for Interuniversity Research Facilities (NCIRF) at Seoul National University. Dissolve the beta-glucan sample in the D2O (Deuterium oxide) at a concentration of 5 mg/mL. HSQC data were acquired on a 2048 × 256-point matrix for the full spectrum, with 64 scans per increment. HMBC data were acquired on a 4096 × 256-point matrix for the full spectrum, with 64 scans per increment.
2.5. Cell culture
Mouse macrophage cell line (RAW 264.7 cells) were purchased from the Korean Cell Line Bank (Seoul, South Korea), and the human spontaneously immortalized keratinocyte cell line (HaCaT) was purchased from the Korea Cell Line Bank. The cells were maintained in DMEM containing 10% FBS and 1% penicillin/streptomycin, under a humidified 5% CO2 atmosphere at 37 °C.
2.6. Cell viability assay and cell morphology assay
The cell viability assay was conducted using the WST-1 solution. LPS is a large molecule consisting of lipid and polysaccharides found in the outer membranes of gram-negative bacteria. Since it is known to stably induce a strong immune response, we used LPS as the positive control. RAW 264.7 cells were plated at a density of 2 × 105 cells per well in 24-well plates and cultured for 24 h. Subsequently, the cells were treated with different concentrations of WSY glucan or 100 ng/mL of LPS for 24 h, and un-treatment group is vehicle as a negative control. After treatment, 10 μL of the cell viability assay solution was added to each well, and the plate was incubated for 2 h. The absorbance was measured with a microplate reader (BioTek Instruments, Inc., Winooski, VT, USA) at a wavelength of 450 nm. Cellular morphology was analyzed after 24 h of incubation and photographed using an inverted microscope (Nikon Inc., Tokyo, Japan).
2.7. Measurement of nitric oxide production
RAW 264.7 cells were plated in 24-well culture plates at 2 × 105 cells per well. The cells were incubated with indicated concentrations of WSY glucan or 100 ng/mL LPS (positive control) for 24 h, and culture supernatants were collected. The nitrite levels were measured to assess nitric oxide production, it was determined by mixing the cell culture supernatant with the same volume of Griess reagent and incubated for 10 min. The absorbance was measured by using spectrometry at a wavelength of 540 nm and calculated by using a sodium nitrite standard curve.
2.8. Measurement of PGE2, TNF-α by enzyme-linked immunosorbent assay (ELISA)
The levels of PGE2 in the RAW 264.7 macrophages cell supernatants was measured by Prostaglandin E2 parameter assay kit, and cytokines (TNF-α and IL-6) was measured by mouse TNF-α, IL-6 Quantikine ELISA kit according to the manufacturer's instructions. RAW 264.7 cells were incubated with indicated concentrations of WSY glucan or 100 ng/mL LPS for 24 h, and culture supernatants were collected and analyzed to measure the levels of PGE2, TNF-α, and IL-6.
2.9. Phagocytosis assay
Phagocytic activity was evaluated using an EZCell Phagocytosis Assay Kit (BioVision, CA, USA), following the manufacturer's instructions. Briefly, macrophages were cultured in a 24-well plate for 24 h, and then treated with WSY glucan at indicated concentrations (10, 50, and 100 μg/mL) for 24 h. After incubation, fluorescein isothiocyanate (FITC)-labeled E.coli were added to the culture medium at a final dilution of 200:1, and the macrophages were stimulated for 1 h. To stop the phagocytosis, the plates were placed on ice for 30 s. The non-phagocytosed E.coli were removed by washing with phosphate-buffered saline (PBS). After that, the macrophages were fixed with 4% paraformaldehyde and stained with rhodamine-phalloidin (5 U/ml) for 2 h to mark the actin cytoskeleton. After washing three times with PBS, the cells were incubated with Hoechst 33342 (Invitrogen, Carlsbad, CA, USA) at a concentration of 1 μg/mL for 10 min. The cells were then examined using an EVOS fluorescence microscope (Advanced Microscopy Group, Bothell, WA, USA.). The numbers of cells were quantified and analyzed with a BD FACS Calibur™ flow cytometer and BD CellQuest™ Pro Software (Becton Dickinson, San Jose, CA, USA).
2.10. Wound healing assay
A scratch wound healing assay was used to determine the wound healing activity of WSY glucan in skin cells. Briefly, 2 × 105 HaCaT cells per well were seeded in a six-well tissue culture plate in DMEM medium containing 10% serum for 24 h. After the cells reached 90% confluence, they were scratched with 1000 μL micropipette tips and washed with PBS to remove the impaired cells. The cells were then incubated with different concentrations of WSY glucan (10, 50, and 100 μg/mL) or 10 ng/mL TGF β1 (positive control) in serum-free medium for 48 h, and an untreated group was used as control group. Cell migration was observed under a microscope (Advanced Microscopy Group, Bothell, WA, USA.) and analyzed by ImageJ software (National Institutes of Health, Bethesda, MD, USA).
2.11. Statistical analysis
All experimental data are presented as the mean ± standard deviation. The differences between the control group and treatment groups were evaluated by one-way ANOVA (SPSS, IBM, Armonk, NY, USA). P-values of <0.05, 0.01, and 0.001 were considered statistically significant.
3. Results
3.1. Identification of low-molecular-weight beta-glucan by HPSEC and NMR spectra
Since Depol™ 667P enzyme commercially available contains a standardized blend of glucanases from strains of Trichoderma sp., the selective enzyme was used to degrade beta-glucans and other insoluble yeast cell wall debris. Furthermore, the heat pre-treatment was carried out for the effective enzyme activity, due to the rigid tertiary structure of beta-1,3/1,6 glucans [15]. As a result of successful hydrolyzation, the aqueous solubility of yeast glucan was increased (Fig. 1A). The effective solubilization was believed to be due to the low-molecular-weight glucan, which is different from the original molecule. Fig. 1B shows the results confirmed by HPSEC analysis, which mostly showed small glucans with molecular weights ranging from 500 to 1300 g/mol after hydrolysis. In addition, beta-(1→6) branched beta-(1→3) glucan was confirmed by NMR analysis (Fig. 1C–F). The peaks between 4.50 and 4.80 ppm in proton NMR spectrum of glucan are attributed to H-1 of glucoses linked beta-(1→3) and (1→6) linkage [16]. The overlapped proton signals from 3.20 ppm to 4.00 ppm are assigned to H2–H6 protons in the sugar backbone, and the proton peaks around 5.40 ppm corresponds to alpha-anomer of the reducing glucose unit.
Fig. 1.
Identification of low-molecular-weight beta-glucan by HPSE C and1H-NMR. (A) The solubility of Yeast beta-glucan (a) and WSY glucan (b) in water (0.5% w/w). (B) HPSEC analysis of WSY glucan with standard references of maltotriose 504, and pullulan 1300 and 6000. (C) Core chemical structure of yeast beta glucan (n = repeating unit), (D) 1H NMR, (E) DEPT-HSQC, and (F) HMBC spectrum of WSY glucan.
Fig. 1E shows the edited HSQC spectrum giving the same information as 13C DEPT-135 experiment where CH and CH3 signals are phased up and CH2 signals are phased down [17]. In the DEPT-HSQC spectra, CH2 and CH peaks are distinguished in red and black. The C-6 carbons of A glucose clearly shifted downfield to 68.85 ppm compared with C-6 carbons (60.41–60.74 ppm) of B and C glucoses with the free OH group, at which point the H-6 of peaks attached to the C-6 carbons. Also, the correlations at 4.78 and 4.54 ppm for 1H and signals between 102.55 and 103.02 ppm for 13C were assigned. In addition, long-range couplings via the glycosidic bonds were assigned by HMBC analysis. As shown in Fig. 1F, a cross peak in a blue rectangle was detected by three-bond correlation between C-6 resonance of A glucose and the H-1 signals of B glucose. The cross peaks by C-3 and H-1 signals of A and C glucose units were also assigned. The NMR data clearly show the presence of beta 1,6 branched beta 1,3 glucan.
3.2. Effect of WSY glucan on the induction of morphological changes in RAW 264.7 macrophage cells
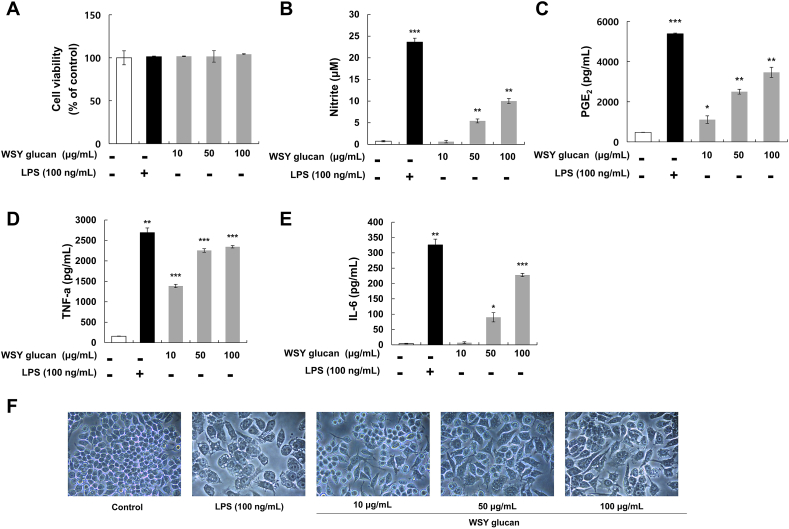
Cell viability was measured using the WST solution and the results demonstrated that WSY glucan had no cytotoxicity to RAW 264.7 cells at concentrations ranging from 10 to 100 μg/mL, which were used in further studies (Fig. 2A). To evaluate the effect of WSY glucan on the production of immune mediators, the production of NO and PGE2, and the pro-inflammatory cytokines IL-6 and TNF-α were measured. As shown in Fig. 2 (B-E), immune mediators release was induced in RAW 264.7 macrophages by WSY glucan in a dose-dependent manner. Fig. 2F presents the cell morphology of macrophage RAW 264.7 cells treated with WSY glucan or LPS (100 ng/mL). After 24 h, the cells were observed and photographed under an optical microscope (400x magnification). In the unstimulated cells (control group), the cell morphology was generally circular, whereas the LPS-activated RAW 264.7 cells changed to an irregular shape while expanding in size and forming villi on the surface. Similarly, compared to the untreated group, WSY glucan treatment significantly increased cell size, as well as spreading, in a concentration-dependent manner (Fig. 2F). Most importantly, the attacked macrophages displayed dendritic contours with microvilli-like structures across their entire surface, indicating definite signs of their immune-boosting ability [18]. These morphological changes suggest that WSY glucan could induce macrophage activity.
Fig. 2.
Effect of WSY glucan on the cell viability, and on the production of inflammatory mediators and pro-inflammatory cytokines and morphology of murine macrophage RAW 264.7 cells. (A) The cell viability assay was measured using WST-1 assay. (B) The levels of nitrite in the culture media were determined using Griess reagent and to reflect NO levels. The levels of (C) PGE2, (D) TNF-α, and (E) IL-6, in the culture medium were measured using an ELISA kit. The results are expressed as the mean ± standard deviation (SD) (n = 3). *p < 0.05, **p < 0.01, and ***p < 0.001 vs. controls. (F) Cellular morphology was assessed after 24 h of incubation and photographed using an inverted microscope (400x magnification).
3.3. Phagocytic activity of WSY glucan in RAW 264.7 cells
Phagocytosis of foreign substance is a key role of macrophages for host defense, which accompanied by change of cell morphology [19]. The effects of WSY glucan on the phagocytic activity of RAW 264.7 cells were investigated by the EZCell phagocytosis assay kit. As shown in Fig. 3A, the immunofluorescence staining results confirmed that the phagocytic activity was increased dose-dependently by WSY glucan treatment. This result was further confirmed using flow chemistry analysis (Fig. 3B–D). The phagocytic activity of cells treated with WSY glucan significantly and dose-dependently increase, and at 100 μg/mL, it was increased by 85% compared to the untreated control cells (Fig. 3D, calculated by mean value). The results indicated that WSY glucan could stimulate the phagocytic activity of macrophages.
Fig. 3.
Phagocytic activity of WSY glucan in RAW 264.7 cells. (A) The phagocytic activity of macrophages was evaluated using the EZCell phagocytosis assay kit and observed by EVOS fluorescence microscopy, the scale bars are 100 μm. (B–D) The phagocytic activity of macrophages determined by flow cytometry. The results are expressed as the mean ± standard deviation (SD) (n = 3). **p < 0.01 and ***p < 0.001 vs. controls.
3.4. Wound healing effect of WSY glucan in HaCaT cells
We examined the effect of WSY glucan on the migration of HaCaT cells using a scratch assay. Platelet derived growth factor (PDGF), epidermal growth factor (EGF), fibroblast growth factor (FGF), and are endogenous growth factors, as is transforming growth factor (TGF)-β1. They are released from some T cells at the site and are presumed to be an essential part of wound healing [20,21]. By measuring the cell migration area in the scratch, the percentage recovery of the treated cells was calculated compared to the controls. As a positive control group, the TGF-β1-treated group showed 199% recovery of the scratched wounds, and the group treated with 100 μg/mL WSY glucan showed 187% recovery (Fig. 4).
Fig. 4.
Wound healing effect of WSY glucan in HaCaT cells. (A) Cell migration was observed using a microscope and (B) analyzed by ImageJ software. The scale bars are 1000 μm. The results are expressed as the mean ± standard deviation (SD) (n = 3). **p < 0.01 and **p < 0.001 vs. controls.
4. Discussion
Recently, yeast-derived beta-glucan has attracted attention for cosmetic, food additive, and medicinal purposes, and has also demonstrated beneficial effects on the outcome of various diseases [22]. However, the use of yeast cell wall-glucan has been limited because it is not soluble in water. In this study, we produced soluble yeast glucan by performing enzymatic hydrolysis. The results of HPSEC analysis confirmed that mostly small glucans with molecular weights ranging from 500 to 1300 g/mol were produced by hydrolysis. These results indicate that the application range of yeast beta-glucan can be broadened by reducing the molecular weight. Molecular weight and branching structures are considered important for biological activity [23]. For beta-glucan found in yeast, it is composed of a (1,3)-beta-linked backbone with a small amount of (1,6)-beta-linked side chains [12,24]. The ability of beta-1,3 and 1,6-glucans to activate innate immunity of cells depends on their branched structure. And beta-1,3-glucan without any side chains (branches) has no effect on activating macrophages [23]. Therefore, the linkage type of WSY glucan was confirmed by 1H-NMR spectral analysis, and the structure of beta-(1→3) and (1→6) bonds was confirmed. In addition, the relative proportion of beta (1→6)-linked glucans were determined to be 52.1% of the total linkages. The rather high content of beta (1→6) bonds was thought to be due to the small cleavage of glucan by the enzymatic reaction.
When activated by various stimuli, the phagocytic activity and ability of macrophages to kill ingested microorganisms was increased and cytokines and immune mediators, modulating cellular immune responses, are produced [25,26]. The present study showed that WSY glucan significantly and dose-dependently potentiated the secretion of pro-inflammatory cytokines (IL-6 and TNF-α) by macrophages and enhanced inflammatory mediator (NO and PGE2) production which play an important role in many physiological processes including immune regulation and the inflammatory response by participating in cell-to-cell communication [27]. Two modes of action may be associated with the effect of beta-glucan immunostimulatory activity on wound healing. One is through indirect activation by various macrophage cytokines, and the other is through direct effects on keratinocytes and fibroblasts [28]. In previous studies, Seo et al. showed that beta-glucan promoted epithelial migration and dermal activation [29]. Cell migration is one of the essential steps in the proliferation phase and wound closure in the wound healing process [30]. Our scratch wound healing assay showed that WSY glucan treatment caused a marked increase in the wound repair capability of the HaCaT cells.
After facing stimuli, the morphology of macrophages is changed due to the development of lamellipodia and filopodia from exterior boundaries, which are hallmarks of the immune response [31]. Thus, to investigate the effect of WSY glucan on macrophage architecture, cell morphological changes were observed after treatment with various doses of WSY glucan. As presented in the results, the WSY glucan at all concentrations tested induced dynamic morphologic changes and enhancement of the phagocytic activity in RAW 264.7 macrophages. When beta-glucan receptors are combined with beta-1,3/1,6 glucan, immune cells including macrophages can improve all immune functions including phagocytosis, the release of certain cytokines such as TNF-α, IL-6, IL-1, interferons, granulocyte-macrophage colony-stimulating factor (GM-CSF), and inflammatory mediators [32]. A previous study reported that yeast beta-glucan enhanced immune activity by binding to the Dectin-1 receptors of macrophages [33,34]. And toll-like receptors (TLRs) were recruited to yeast-containing phagosomes where they detected fungal components and induced the production of pro-inflammatory cytokines [35]. In this research, we did not explore the role of WSY glucan in signal transduction and which receptors were involved in enhancing immunity. Therefore, it is necessary to study the mechanism of WSY glucan in enhancing macrophage immunity further.
In this study, we produced a soluble low-molecular-weight beta-glucan, WSY glucan, and confirmed various aspects of its immune-enhancing effects. Based on the results, WSY glucan demonstrated the potential as a candidate for the treatment of immune weakening-related diseases without solubility limitations. However, it is necessary to further determine the therapeutic effect of WSY glucan as an immune enhancer in established animal models and clinical trials.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
No form of funding was received for this study.
Data availability
Data will be made available on request.
References
- 1.Říhová B., Kovář L., Kovář M., Hovorka O. Cytotoxicity and immunostimulation: double attack on cancer cells with polymeric therapeutics. Trends Biotechnol. 2009;27:11–17. doi: 10.1016/j.tibtech.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Aspinall R., Del Giudice G., Effros R.B., Grubeck-Loebenstein B., Sambhara S. Challenges for vaccination in the elderly. Immun. Ageing. 2007;4:1–9. doi: 10.1186/1742-4933-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poh A.R., Ernst M. Targeting macrophages in cancer: from bench to bedside. Front. Oncol. 2018;8:49. doi: 10.3389/fonc.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beutler B. Innate immunity: an overview. Mol. Immunol. 2004;40:845–859. doi: 10.1016/j.molimm.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Medzhitov R., Janeway C.A., Jr. Innate immunity: impact on the adaptive immune response. Curr. Opin. Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- 6.Kim H.J., Lee J., Kim S.C., Seo J.Y., Hong S.B., Park Y.I. Immunostimulating activity of Lycium chinense Miller root extract through enhancing cytokine and chemokine production and phagocytic capacity of macrophages. J. Food Biochem. 2020;44 doi: 10.1111/jfbc.13215. [DOI] [PubMed] [Google Scholar]
- 7.Steinhoff M., Brzoska T., Luger T.A. Keratinocytes in epidermal immune responses. Curr. Opin. Allergy Clin. Immunol. 2001;1:469–476. doi: 10.1097/01.all.0000011062.60720.e3. [DOI] [PubMed] [Google Scholar]
- 8.Piipponen M., Li D., Landén N.X. The immune functions of keratinocytes in skin wound healing. Int. J. Mol. Sci. 2020;21:8790. doi: 10.3390/ijms21228790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tokunaka K., Ohno N., Adachi Y., Tanaka S., Tamura H., Yadomae T. Immunopharmacological and immunotoxicological activities of a water-soluble (1→3)-β-d-glucan, CSBG from Candida spp. Int. J. Immunopharm. 2000;22:383–394. doi: 10.1016/s0192-0561(99)00093-4. [DOI] [PubMed] [Google Scholar]
- 10.Stier H., Ebbeskotte V., Gruenwald J. Immune-modulatory effects of dietary yeast beta-1, 3/1, 6-D-glucan. Nutr. J. 2014;13:1–9. doi: 10.1186/1475-2891-13-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novak M., Vetvicka V. β-glucans, history, and the present: immunomodulatory aspects and mechanisms of action. J. Immunot. 2008;5:47–57. doi: 10.1080/15476910802019045. [DOI] [PubMed] [Google Scholar]
- 12.Bohn J.A., BeMiller J.N. (1→ 3)-β-d-Glucans as biological response modifiers: a review of structure-functional activity relationships. Carbohydr. Polym. 1995;28:3–14. [Google Scholar]
- 13.Ahmad A., Anjum F.M., Zahoor T., Nawaz H., Dilshad S.M.R. Beta glucan: a valuable functional ingredient in foods. Crit. Rev. Food Sci. Nutr. 2012;52:201–212. doi: 10.1080/10408398.2010.499806. [DOI] [PubMed] [Google Scholar]
- 14.Samuelsen A.B.C., Schrezenmeir J., Knutsen S.H. Effects of orally administered yeast‐derived beta‐glucans: a review. Mol. Nutr. Food Res. 2014;58:183–193. doi: 10.1002/mnfr.201300338. [DOI] [PubMed] [Google Scholar]
- 15.Kumagai Y., Okuyama M., Kimura A. Heat treatment of curdlan enhances the enzymatic production of biologically active β-(1, 3)-glucan oligosaccharides. Carbohydr. Polym. 2016;146:396–401. doi: 10.1016/j.carbpol.2016.03.066. [DOI] [PubMed] [Google Scholar]
- 16.Lowman D.W., West L.J., Bearden D.W., Wempe M.F., Power T.D., Ensley H.E., Haynes K., Williams D.L., Kruppa M.D. New insights into the structure of (1→ 3, 1→ 6)-β-D-glucan side chains in the Candida glabrata cell wall. PLoS One. 2011;6 doi: 10.1371/journal.pone.0027614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choudhary M.I. Academic Press; 2015. Solving Problems with NMR Spectroscopy. [Google Scholar]
- 18.Khatua S., Chandra S., Acharya K. Hot alkali‐extracted antioxidative crude polysaccharide from a novel mushroom enhances immune response via TLR‐mediated NF‐κB activation: a strategy for full utilization of a neglected tribal food. J. Food Biochem. 2021;45 doi: 10.1111/jfbc.13594. [DOI] [PubMed] [Google Scholar]
- 19.Freeman S.A., Grinstein S. Phagocytosis: how macrophages tune their non-professional counterparts. Curr. Biol. 2016;26:R1279–R1282. doi: 10.1016/j.cub.2016.10.059. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y., Dulchavsky D.S., Gao X., Kwon D., Chopp M., Dulchavsky S., Gautam S.C. Wound repair by bone marrow stromal cells through growth factor production. J. Surg. Res. 2006;136:336–341. doi: 10.1016/j.jss.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 21.Strutz F., Zeisberg M., Renziehausen A., Raschke B., Becker V., Van Kooten C., Müller G.A. TGF-β1 induces proliferation in human renal fibroblasts via induction of basic fibroblast growth factor (FGF-2) Kidney Int. 2001;59:579–592. doi: 10.1046/j.1523-1755.2001.059002579.x. [DOI] [PubMed] [Google Scholar]
- 22.Rop O., Mlcek J., Jurikova T. Beta-glucans in higher fungi and their health effects. Nutr. Rev. 2009;67:624–631. doi: 10.1111/j.1753-4887.2009.00230.x. [DOI] [PubMed] [Google Scholar]
- 23.Akramienė D., Kondrotas A., Didžiapetrienė J., Kėvelaitis E. Effects of ß-glucans on the immune system. Medicina. 2007;43:597. [PubMed] [Google Scholar]
- 24.Raa J. Immune modulation by non-digestible and non-absorbable beta-1, 3/1, 6-glucan. Microb. Ecol. Health Dis. 2015;26:27824. doi: 10.3402/mehd.v26.27824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guzik T., Korbut R., Adamek-Guzik T. Nitric oxide and superoxide in inflammation. J. Physiol. Pharmacol. 2003;54:469–487. [PubMed] [Google Scholar]
- 26.Kim G.S., Nalini M., Kim Y., Lee D.W. Octopamine and 5‐hydroxytryptamine mediate hemocytic phagocytosis and nodule formation via eicosanoids in the beet armyworm, Spodoptera exigua. Arch. Insect Biochem. Physiol.: Publ. Collaboration Entomol. Soc. Am. 2009;70:162–176. doi: 10.1002/arch.20286. [DOI] [PubMed] [Google Scholar]
- 27.Bellanti J.A., Kadlec J.V., Escobar-Gutiérrez A. Cytokines and the immune response. Pediatr. Clin. 1994;41:597–621. doi: 10.1016/s0031-3955(16)38800-9. [DOI] [PubMed] [Google Scholar]
- 28.Majtan J., Jesenak M. β-Glucans: multi-functional modulator of wound healing. Molecules. 2018;23:806. doi: 10.3390/molecules23040806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seo G., Hyun C., Choi S., Kim Y.M., Cho M. The wound healing effect of four types of beta-glucan. Appl. Biol. Chem. 2019;62:1–9. [Google Scholar]
- 30.Krawczyk W.S. A pattern of epidermal cell migration during wound healing. J. Cell Biol. 1971;49:247–263. doi: 10.1083/jcb.49.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Venter G., Oerlemans F.T., Wijers M., Willemse M., Fransen J.A., Wieringa B. Glucose controls morphodynamics of LPS-stimulated macrophages. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meena D., Das P., Kumar S., Mandal S., Prusty A., Singh S., Akhtar M., Behera B., Kumar K., Pal A. Beta-glucan: an ideal immunostimulant in aquaculture (a review) Fish Physiol. Biochem. 2013;39:431–457. doi: 10.1007/s10695-012-9710-5. [DOI] [PubMed] [Google Scholar]
- 33.Brown G.D., Taylor P.R., Reid D.M., Willment J.A., Williams D.L., Martinez-Pomares L., Wong S.Y., Gordon S. Dectin-1 is a major β-glucan receptor on macrophages. J. Exp. Med. 2002;196:407–412. doi: 10.1084/jem.20020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor P.R., Tsoni S.V., Willment J.A., Dennehy K.M., Rosas M., Findon H., Haynes K., Steele C., Botto M., Gordon S. Dectin-1 is required for β-glucan recognition and control of fungal infection. Nat. Immunol. 2007;8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Underhill D.M., Ozinsky A., Hajjar A.M., Stevens A., Wilson C.B., Bassetti M., Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.