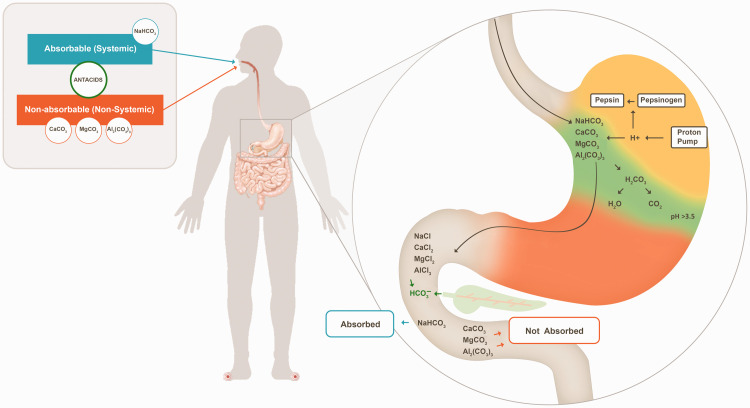
Figure 1.
Effects of different antacid ingredients on gastric acid. The representative figure presents the mechanism of carbonate salts only. Other antacid salts were discussed in the article. Most of the gastric acid (approximately 45 mEq/h) is secreted across the apical membrane of the stomach through a proton pump (H+/K+ ATPase) after meal consumption. The carbonate salt of antacids binds to H+ ions from gastric hydrochloric acid to produce chloride salts (calcium chloride, sodium chloride, magnesium chloride, and aluminum chloride), carbon dioxide, and water. This decreases H+ concentrations in the stomach, thus raising the pH. The orange region denotes the acidic environment of the stomach, the green region denotes the antacid-mediated neutralization/adsorption of gastric acid, and the yellow region denotes alkalized/neutralized gastric acid. In the alkaline conditions of the small intestine, soluble calcium chloride, sodium chloride, magnesium chloride, and aluminum chloride are converted back to their carbonate salts. The sodium bicarbonate rapidly empties into the small intestine, where it is absorbed; thus, it is considered an absorbable antacid. Calcium carbonate, magnesium carbonate, and aluminum carbonate are excreted with the stool, decreasing their absorption; thus, they are considered non-absorbable antacids.