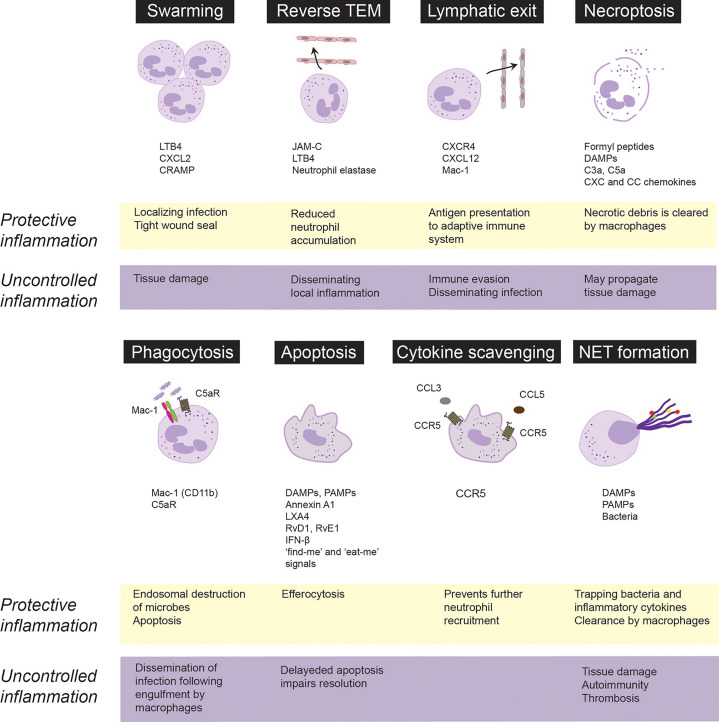
Figure 1.
Fate and roles of emigrated neutrophils in protective vs. uncontrolled inflammation. Neutrophils are rapidly recruited from the circulation to the infected or injured tissues. Following extravasation, neutrophils swarm toward the infected sites to localize infection and from a tight wound seal. Neutrophils may trap, neutralize and kill invading pathogens through necroptosis, phagocytosis or release of extracellular traps (NETs). Phagocytosis of opsonized bacteria usually induces apoptosis followed by phagocytosis of apoptotic cells by macrophages via efferocytosis. Neutrophils integrate pro-survival and apoptosis-promoting cues from the inflammatory environment, which governs their lifespan. Apoptotic neutrophils express CCR5, which by binding chemokines prevents further neutrophil recruitment. Excessive swarming and necroptosis may aggravate and perpetuate tissue damage. Neutrophils may egress from the inflammatory locus through reverse transendothelial migration (TEM) or lymphatic vessels, which dampen neutrophil accumulation, but may also lead to immune evasion and dissemination of local inflammation. Neutrophils carrying bacteria that they cannot destroy, may serve as “Trojan horses” to disseminate the infection on phagocytosis of apoptotic neutrophils by macrophages. CRAMP, cathelin-related antimicrobial peptide; DAMPs, damage-associated molecular patterns, JAM-C, junctional adhesion molecules C; LTB4, leukotriene B4; LXA4, lipoxin A4; PAMPs, pathogen-associated molecular patterns; RvD1, resolvin D1; RvE1, resolvin E1.