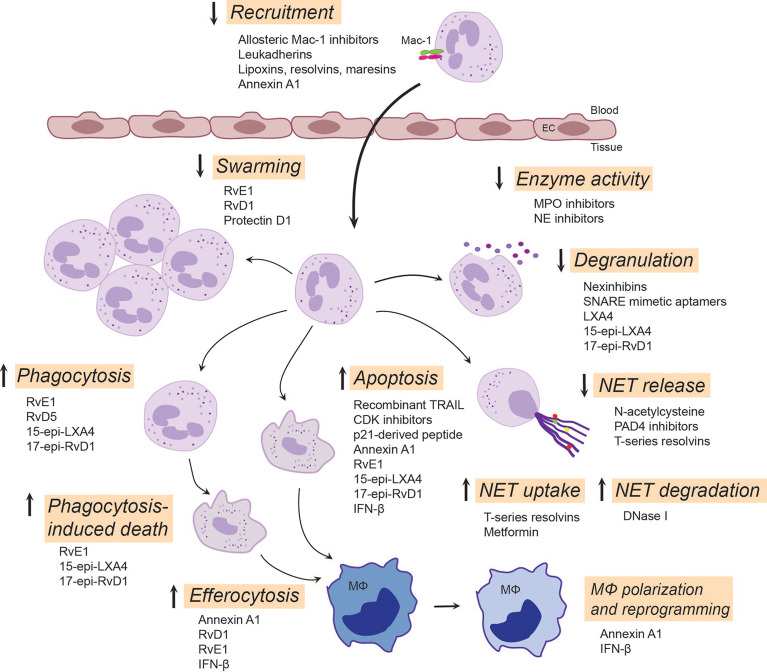
Figure 2.
Emerging neutrophil-targeted therapeutic approaches to promote the resolution of inflammation. The strategies include blocking, restoring or activating neutrophil functions. Thus, blocking function of Mac-1 or upregulation of Mac-1expression dampens neutrophil accumulation, a critical component of terminating the inflammatory response. LXA4, RvE3 and protectin D1 serve as stop signals for swarming. Inhibition of degranulation or the activity of secreted enzymes, such as MPO and NE, could reduce tissue injury and alter composition of NETs. Enhancing NET degradation by DNase I or promoting NET uptake by T-series resolvins or metformin may prevent the deleterious actions of excessive NET formation. RvD5 and RvE1 facilitates phagocytosis, whereas 15-epi-LXA4 and 17-epi-RvD1 restore impaired phagocytosis, facilitate clearance of bacteria and phagocytosis-induced apoptotic cell death. By countering survival cues, many molecules, including CDK inhibitors, annexin A1, IFN-β and lipid SPMs, can redirect neutrophils to apoptosis and promote their uptake by macrophages through efferocytosis. This leads to reprogramming and polarization of macrophages toward a pro-resolution, regenerative phenotype that promotes further removal of neutrophils. Annexin A1, RvE1, RvD1 and IFN-β play pivotal roles in mediating feedforward resolution programs. Of note, although most of these data are from experimental models, some strategies (e.g. LXA4 mimetics, NE inhibitors or DNase I) are currently being investigated in clinical trials. C5aR, complement C5a receptor; CDK, cyclin-dependent kinase; EC, endothelial cell; IFN-β, interferon-β; LXA4, lipoxin A4; 15-epi-LXA4, 15-epi-lipoxin A4; MPO, myeloperoxidase; NE, neutrophil elastase; NET, neutrophil extracellular traps; PAD4, peptidyl arginine deiminase 4; 17-epi-RvD1, 17-epi-resolvin D1; RvE1, resolvin E1; RvE3, resolvin E3; RvD5, resolvin D5; SNARE, soluble N-ethylmaleimide-sensitive-factor attachment protein receptor; SPMs, specialized pro-resolving mediators; TRAIL, TNF-related apoptosis-inducing ligand.