Fig. 3.
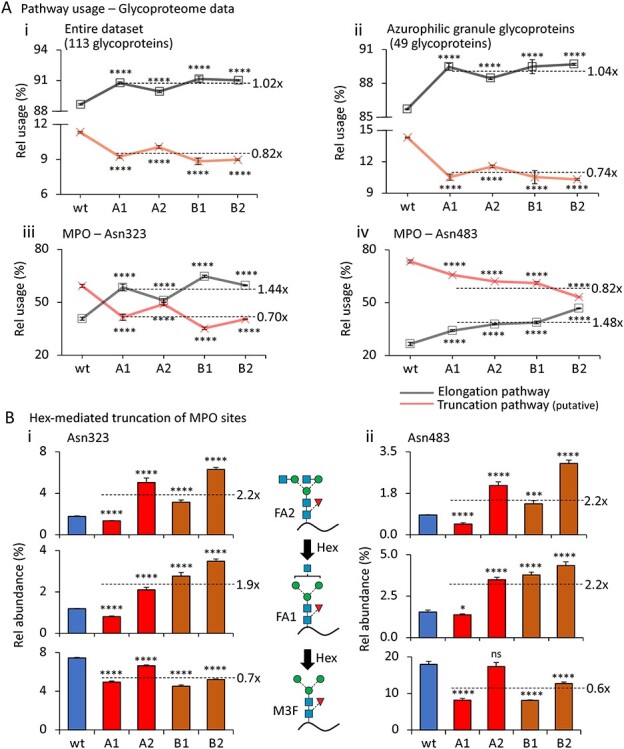
Hex disruption reduces paucimannosylation of azurophilic granule-resident proteins in the HL-60 N-glycoproteome. (A), Relative utilization of the proposed truncation and elongation pathway (see Figure 2C for schematics) based on all observed N-glycopeptides carrying N-glycans mapping to the two pathways arising from (i) the entire HL-60 glycoproteome, (ii) HL-60 source proteins known to traffic to and reside in the azurophilic granule (broken line represents average fold change relative to wt) (Rorvig et al. 2013), and (iii) Asn323 and (iv) Asn483 of MPO, a prominent azurophilic granule-resident glycoprotein. (B), Relative abundance of the GlcNAc-capped Hex substrates (FA1 and FA2, top graphs) and immediate Hex product (M3F, bottom graphs) decorating (i) Asn323 and (ii) Asn483 of MPO. The mean relative abundance for all HL-60 mutants (A1, A2, B1, B2, broken lines) and the fold change relative to the unedited wt are provided for each of the three N-glycan structures quantified across the two sites. For all panels, data have been plotted as mean ± SD, n = 3 technical replicates, ns, not significant, *P < 0.05, ***P < 0.005, ****P < 0.001 (mutant vs wt HL-60). See Figure 2 for glycan symbol and linkage representation (Neelamegham et al. 2019).
