Abstract
Background and Objectives
Cognitive resilience is a well-recognized concept, but knowledge gaps about its underlying mechanisms have made it difficult to develop instruments that identify older adults with high or low resilience. We tested whether aggregating cortical peptides associated with cognitive resilience into an index can identify adults with higher or lower cognitive resilience.
Methods
We used data from 1,192 older decedents, including annual clinical testing, indices of 10 Alzheimer disease (AD) and related dementia (ADRD) pathologies, and 226 proteotypic peptides measured in the dorsal lateral prefrontal cortex. We used linear mixed-effects models to identify peptides that were related to cognitive resilience (i.e., cognitive decline not explained by ADRD pathologies [false discovery rate <0.05]). We aggregated the expression levels of these resilience peptides into a person-specific cognitive resilience index and examined its association with AD clinical and pathologic phenotypes.
Results
We constructed a resilience index from 52 of 226 peptides related to cognitive resilience. A higher index was associated with slower cognitive decline (estimate 0.05, SE 0.003, p < 0.001) and slower motor decline (estimate 0.005, SE 0.001, p < 0.001). Most resilience peptides (70%) were specific to cognitive decline, but 30% also provided resilience for motor decline. A higher index was also related to a lower burden of AD pathologies (odds ratio [OR] 0.41, SE 0.01, p < 0.001) and modified the association of AD pathology with cognition in that a higher index modified the negative effects of AD pathology on AD dementia proximate to death (OR 0.70, SE 0.14, p = 0.010). Up to 90% of cognitive resilience peptides were related to AD pathologic phenotypes.
Discussion
Cortical proteins may provide some degree of cognitive resilience. These multifunctional proteins also seem to provide resilience to other AD clinical phenotypes and have independent associations with ADRD pathologies. Resilience proteins may be high-value therapeutic targets for drug discovery of interventions that maintain brain health in aging adults via multiple pathways.
Many reports suggest that the heterogeneity of cognitive decline in older adults may be due, in part, to the fact that some individuals are more resilient to Alzheimer disease (AD) and related dementia (ADRD) pathologies than others.1-4 Cognitive resilience is often conceptualized as slower-than-expected cognitive decline despite the presence of brain injury or pathologies.5,6 It is hypothesized that cognitive resilience emerges from diverse factors, including structural factors, biological factors, or behaviors acquired over the lifespan that offset the negative effects (i.e., cognitive decline) of accumulating ADRD pathologies.5,6
Several different mechanisms have been hypothesized to account for cognitive resilience that are thought to be dependent on functional and structural brain processes.1,7 Neurobiological capital, called brain reserve, can provide some degree of resilience, depending on individual differences in brain structure and anatomy.1,7 Acquired cognitive skills and abilities called cognitive reserve may also provide resilience to brain aging, insult, or pathology via the efficiency, capacity, and flexibility of one's cognitive systems that help to explain such susceptibility.1,8-11 Yet, there is a paucity of data about the underlying molecular mechanisms of these distinct but related complex constructs, which has made it difficult to translate these phenotypes into instruments that can be used to identify older adults with high or low cognitive resilience.6,7,12
Attempting to elucidate underlying biological mechanisms that differentiate and identify adults with high or low cognitive resilience is vital to inform the homogeneity of adults needed in clinical trials for ADRD interventions and risk stratification for allocating interventions to vulnerable older adults who might benefit from early treatments. The application of proteomics to investigate cognitive resilience is a novel approach that attempts to explicate the biological mechanisms associated with cognitive resilience. The progression toward a quantifiable measure of cognitive resilience has the potential to advance the field on both theoretical and empirical grounds. Resilience proteins may be high-value therapeutic targets for drug discovery of personalized resilience therapies that offset the negative cognitive effects of untreatable ADRD pathologies.12
Recently, we interrogated a clinical measure of cognitive resilience (i.e., cognitive decline not explained by ADRD pathologies, and identified genes13 and proteins that may provide cognitive resilience.5,6 These reports used either proteome-wide tandem mass tag proteomics8 or selective reactive monitoring (SRM) targeted proteomics14 to identify a parsimonious group of cognitive resilience proteins. Some cortical resilience proteins were associated with slower cognitive decline, while others were associated with faster cognitive decline. Proteins identified in these discovery studies have been validated in further studies.13 No study has yet aggregated multiple cortical resilience proteins into a single index that may summarize person-specific indices of resilience.
Building on our prior work,5,6,14 we used clinical and postmortem data from >1,200 older decedents from 2 independent longitudinal cohorts. We isolated cognitive resilience, i.e., cognitive decline unrelated to the negative effects of ADRD pathologies. We then aggregated the expression level of SRM proteins that remained associated with cognitive resilience into a person-specific resilience index. We then tested whether the constructed resilience index could be used to identify individuals with higher- or lower-than-average cognitive resilience.
Methods
Study Participants
Participants were community-based older adults enrolled in 2 ongoing cohort studies of aging and dementia, the Religious Orders Study (ROS)15 and the Rush Memory and Aging Project (MAP).16 On enrollment, participants agreed to annual clinical testing and to brain donation after death. Uniform annual clinical testing and postmortem assessments are performed in both studies by the same staff, facilitating joint analyses. Participants had no known dementia on enrollment.
Assessment of Cognitive Function and Cognitive Status Diagnoses
Detailed annual neuropsychological assessment and clinical examinations were administered.17 A global composite score was derived by standardizing 17 tests assessing 5 domains of cognitive function using baseline means and SDs of both cohorts. Repeated cognitive measures were used to estimate the rate of cognitive decline before death.
A 3-step process based on algorithms and clinical judgment was applied to diagnose AD dementia and other dementias, as described previously.16,18 Final cognitive status was provided by a neurologist at the time of death and after a review of all clinical data.19
Other Clinical Covariates
Demographic measures such as age, sex, and number of years in formal education are recorded at enrollment, and age at death is calculated from the date of death and date of birth.20 A global motor score summarized 10 common performances, as described previously.21 Parkinsonism was quantified with the composite measure of 26 items from a modified version of the motor portion of the United Parkinson's Disease Rating Scale.21
Assessment of ADRD Pathologies
On death, the brain was removed and hemisected following standard procedure.22 One hemisphere was prepared for histologic evaluation, and the other hemisphere was frozen. The fresh slabs were fixed in 4% paraformaldehyde. The hemisphere was cut into 1-cm coronal slabs. Tissue blocks from predetermined regions were dissected, embedded in paraffin, and cut into 6- and 20-µm sections.22 Measured indices included (1) AD pathology, (2) hippocampal sclerosis, (3) TAR DNA-binding protein 43 (TDP-43), (4) nigral neuronal loss, (5) Lewy bodies, (6) macroinfarcts, (7) microinfarcts, (8) cerebral angiopathy, (9) atherosclerosis, and (10) arteriolosclerosis.23 Neuropathologic data collection and assessment were performed by investigators blinded to all clinical and cognitive data, as previously described.24,25
Targeted SRM Proteomics
Targeted proteomic analysis was performed with frozen tissue from the dorsolateral prefrontal cortex as part of the Accelerating Medicines Partnership–AD Consortium study, following standard protocol.5 The dorsal lateral prefrontal cortex was chosen on the basis of a large body of work that has identified the prefrontal cortex as a crucial region for cognitive control and for translating ideas or goals into varied behaviors.11,26-28 It is a region well developed in humans who get AD and can be devoid of any brain pathology as opposed to mesial temporal regions, which are typically riddled with 2 or 3 pathologies with virtually no cases without any pathology.29,30A total of 126 genes and a corresponding 226 proteotypic peptides were nominated on the basis of prior literature and research suggesting an association with AD/ADRD clinical and pathologic phenotypes. More detailed information can be found in eAppendix 1 (links.lww.com/WNL/B808).
Statistical Analysis
Cognitive Resilience Proteins
First, we ran growth linear mixed-effects models allowing random effects on both the intercept and the slope and adjusting for age at death, sex, and education to investigate person-specific annual rate of cognitive decline (model 1), with the individuals being the random effect, the predictors and covariates being fixed independent variables, and the slope of cognitive decline leading up to death being the outcome. None of the variation around the slope was significant in any of the models. Then, we added individual peptides to model 1; i.e., we ran a total of 226 models (separate models for each peptide) to identify proteotypic peptides associated with cognitive decline (model 2). Last, in each of the 226 models, we further adjusted for 10 ADRD pathologic indices (model 3); this enabled us to investigate peptides associated with cognitive resilience, i.e., cognitive decline not explained by ADRD pathologies. The latter peptides, which are associated with cognitive decline after adjustment for ADRD pathologies in model 3, are called cognitive resilience proteins. All p values were adjusted using false discovery rate (FDR p < 0.05) to correct for multiple testing.
Cognitive Resilience Index
We multiplied the expression level for each resilience protein by its effect size (i.e., estimate for interaction with annual rate of cognitive decline after controlling for brain pathologies) for each participant. This was repeated for each of the 52 peptides. The 52 scores from each of the peptides were then averaged into a single score for every participant. This was then z scored for standardization purposes and called the cognitive resilience index (CRI).
CRI and AD Clinical Phenotypes
We repeated linear mixed-effect models to investigate the person-specific rate of annual cognitive decline as predicted by the constructed CRI, adjusting for demographics and 10 ADRD pathologies. In a final step, we examined a series of logistic regression models to test whether a higher CRI is related to lower odds of mild cognitive impairment (MCI) and AD dementia proximate to death. We also used linear mixed-effect models to examine the association of CRI with declining motor function, a noncognitive ADRD clinical phenotype, using global motor score and parkinsonism before death.
CRI and ADRD Pathologies
We used regression models to examine the associations of the constructed CRI with ADRD pathologies, including the presence of pathologic AD according to National Institute on Aging–Reagan criteria and associations with tau tangles and β-amyloid. We added interaction terms to a regression model to examine whether the constructed CRI modified the known association of AD pathology and cognition. We also examined a series of regression models to determine which of the identified cognitive resilience proteins were related to each of the 3 AD pathologic phenotypes (AD pathology, β-amyloid, tau). In secondary analyses, we also modeled the associations of the CRI on non-AD pathologic phenotypes. All models were adjusted for age at death, sex, and education. All analyses were carried out with SAS software (SAS Institute Inc, Cary, NC).
Standard Protocol Approvals, Registrations, and Patient Consents
Both studies were approved by an Institutional Review Board of Rush University Medical Center. All study participants provided written informed consent and an Anatomical Gift Act for organ donation.
Data Availability
All data included in these analyses are available the Rush Alzheimer's Disease Center Research Resource Sharing Hub.31 Descriptions of the studies and data can be found in this hub. Qualified investigators may create an account and submit requests for deidentified data.
Results
Descriptive Characteristics of the Study Participants
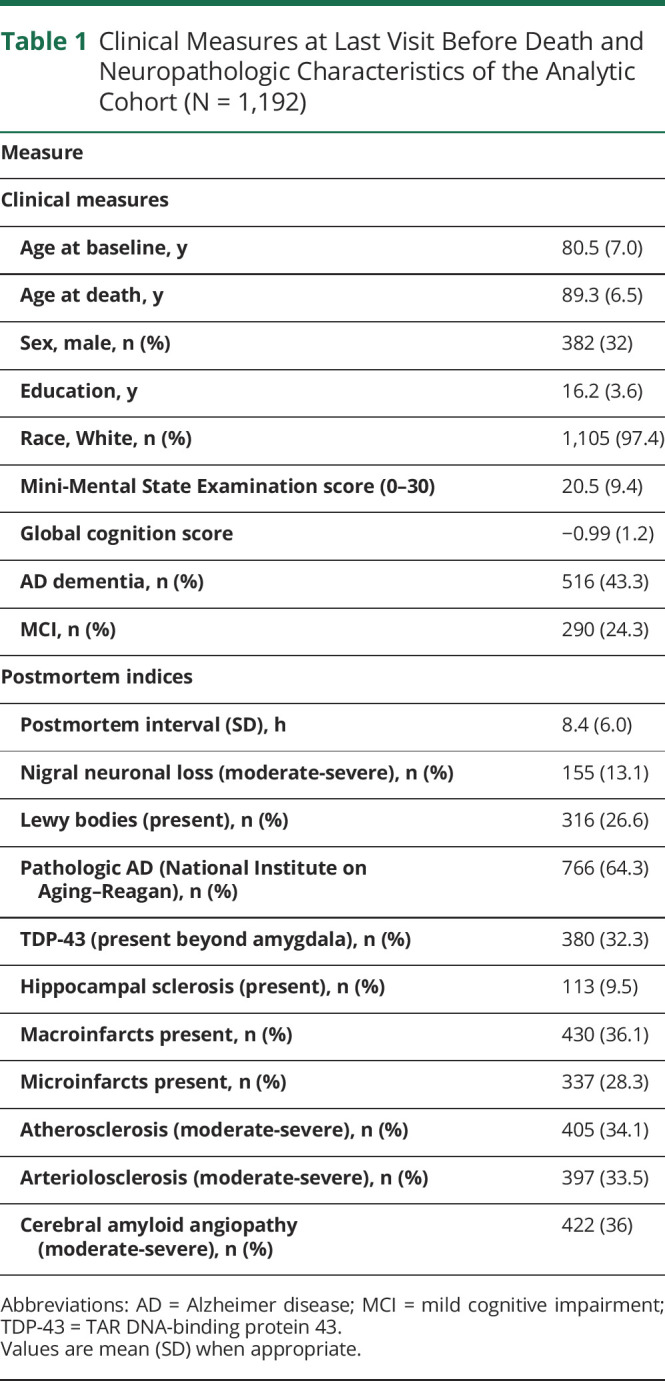
A total of 1,192 decedents (ROS n = 547, 45.9%, MAP n = 645, 54.1%) with a mean of 8.7 (SD 4.5) years of follow-up were included in this study. Their clinical characteristics at their last visit proximate to death and postmortem indices are summarized in Table 1.
Table 1.
Clinical Measures at Last Visit Before Death and Neuropathologic Characteristics of the Analytic Cohort (N = 1,192)
Identifying Cognitive Resilience Proteins
We examined 226 separate linear mixed-effects models that were adjusted for age at death, sex, and education. Of these, 110 peptides were associated with the rate of cognitive decline (FDR adjusted at p < 0.05). Fifty-two of 110 peptides remained associated with cognitive decline, controlling for pathology. Of those, higher levels of 31 peptides and lower levels of 21 peptides were associated with slower cognitive decline independently of ADRD pathology. Figure 1 illustrates these associations. eTable 1 (links.lww.com/WNL/B808) lists each of the estimates of these peptides, p values, and standard errors. eTable 2 lists each of the expression means and range of variances of the peptides.
Figure 1. Fifty-Two Peptides Are Independently Associated With Cognitive Resilience.
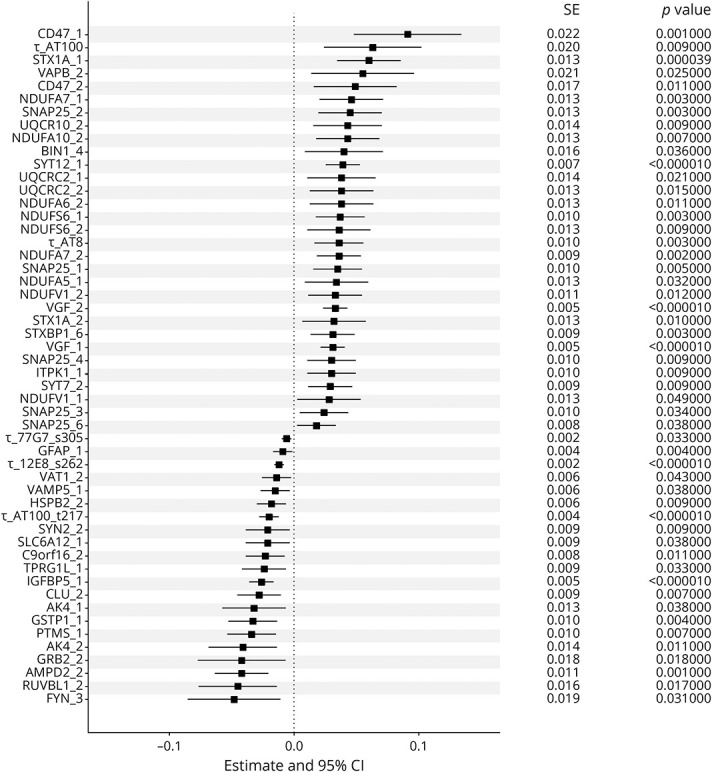
Individual peptides measured in the dorsal lateral prefrontal cortex are represented by squares. Each row shows results for the interaction of a single peptide with the annual rate of cognitive decline for an average of ≈9 years of follow-up. This term derives from a linear mixed-effect model that included 15 terms, including time (annual rate of cognitive decline), age, sex, education, expression level of a single peptide, and terms for each of the 10 brain pathologies, as well as their interaction with time. Squares on the left from 0 represent a negative association with cognitive decline, i.e., faster cognitive decline for higher levels of protein; squares on the right from 0 represent a positive association with cognitive decline, i.e., slower cognitive decline for a higher level of protein. Bars show 95% CIs. The y-axis on the right displays the standard errors (SEs) and p values of individual peptides (false discovery rate <0.05).
We reviewed the functions of the 52 identified proteins and found that the majority of the functions were mitochondrial and synaptic plasticity (eTable 3, column 2, links.lww.com/WNL/B808). Next, we examined an exploratory principal components analysis to determine whether the 52 proteins might cluster in factors that share common physiologic functions to provide resilience. We retained 10 factors with eigenvalues >1 and proteins with loadings >0.50. Two-thirds of the proteins clustered in the first 3 factors. The first component was driven mainly by mitochondrial proteins; the second component consisted of synaptic proteins; and the third component was driven by proteins involved in cell structure and function. Full details of this analysis are provided in eTable 3.
Constructing a CRI
On the basis of each participant's expression level for each of the 52 proteins that remained associated with cognitive decline after controlling for pathology, we calculated a person-specific index as described above. As expected, on the basis of its construction, the index was associated with the rate of cognitive decline, with a higher resilience index being associated with a slower rate of cognitive decline (estimate 0.05, SE 0.003, p < 0.001). The index was not associated with age (r = 0.03, p = 0.3) or education (r = −0.00006, p = 0.998). There was no difference between men and women on the index (t = −0.05 p = 0.9).
To assess whether CRI is independently associated with cognitive decline, we calculated the percentage of variance of cognitive decline accounted for CRI and brain pathologies. First, in separate models relative to terms for demographics alone, CRI accounted for 19.2% of additional variance of cognitive decline, and brain pathologies accounted for 26.7% of additional variance of cognitive decline. Then, in the single joint model with both CRI and ADRD pathologies in the model, ARDR pathologies accounted for 22.7% of variance of cognitive decline, and CRI accounted for 11.8% of variance. This suggests that the resilience index of aggregated proteins has an independent positive association with cognitive decline that is separate from the well-known negative association of ADRD pathologies with cognitive decline.
We also recalculated the resilience index separately for the 2 independent samples of older adults analyzed in this study, i.e., the ROS and MAP cohorts. The Pearson correlation for the resilience indices between the 2 cohorts was 0.89, indicating the replicability and robustness of the index.
CRI and AD Clinical Phenotypes
Cognitive AD Phenotypes
In separate regression models, with cognitive status (MCI, AD dementia) as the outcome, a higher CRI was related to lower odds of MCI (odds ratio [OR] 0.45, 95% CI 0.40–0.51, p < 0.001) and AD dementia (OR 0.41, 95% CI 0.35–0.47, p < 0.001) proximate to death. On further adjustment for pathology, CRI remained associated with lower odds of MCI (OR 0.70, 95% CI 0.57–0.86, p < 0.001) and AD dementia (OR 0.66, 95% CI 0.51–0.85, p < 0.01) proximate to death.
Noncognitive ADRD Phenotypes
To examine the specificity of CRI for cognitive resilience, we repeated the linear mixed-effects model described above, replacing the outcome of cognitive decline with global motor decline and with progressive Parkinsonism.32 CRI was associated with slower rate of global motor decline (estimate 0.005, SE 0.0009, p < 0.001) and with slower rate of progressive Parkinsonism (estimate −0.022, SE 0.0045, p < 0.001).
Figure 2, A–C illustrates model-derived trajectories for rate of cognitive decline (top), motor decline (middle), and progressive Parkinsonism (bottom) for 3 representative participants (female, 90 years old, with 15 years of education), with high (90th percentile), average (50th percentile), and low (10th percentile) CRIs. The individual with a high resilience index manifests a slower (less steep) trajectory of cognitive decline (black line) compared to an individual with a low resilience score (red line).
Figure 2. CRI and Late-Life Cognitive and Motor Impairment.
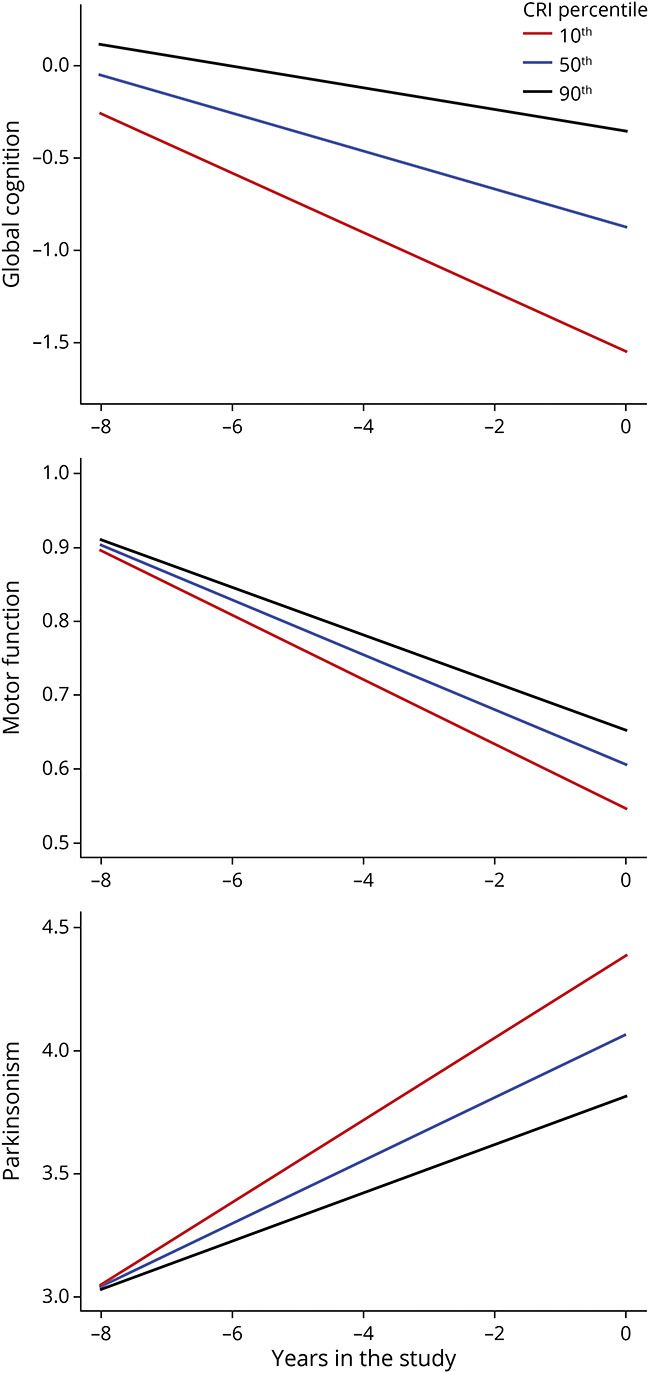
Model derived trajectories for 3 average participants: 90-year-old woman with 15 years of education contrasting rate of decline for low (10th percentile, red line), average (50th percentile, blue line), and high (90th percentile, black line) cognitive resilience index (CRI). An individual with low vs high CRI showed 2.6-fold faster rate of cognitive decline, 1.33-fold faster rate of motor decline, and 1.31-fold faster rate of progressive parkinsonism.
Noncognitive AD Phenotypes and Individual Cognitive Resilience Proteins
To determine whether any of the 52 peptides associated with cognitive resilience provide resilience for other aging phenotypes implicated in ADRD, we performed a complementary analysis of the associations of the 226 SRM proteins with motor resilience, i.e., motor decline manifested by repeated measures of global motor scores not explained by brain pathologies. We found that 14 of the 52 proteins (26.9%) used to construct the CRI were also associated with motor resilience (eTable 4, links.lww.com/WNL/B808).
We performed a similar analysis examining the associations of these peptides with a second motor phenotype, progressive parkinsonism. We found that 10 of the 52 proteins (19%) used to construct the index were also associated with residual progressive parkinsonism, i.e., progression not explained by 10 brain pathologies (eTable 4, links.lww.com/WNL/B808). Combining the results of these 3 complementary analyses shows that most (∼70%) of the 52 cognitive resilience proteins are specific for cognitive decline and ∼30% may provide resilience for both cognitive and noncognitive AD phenotypes (Figure 3 and eTable 4).
Figure 3. Some Cortical Proteins May Provide Resilience for a Specific Aging Phenotype, and Some Provide Resilience for >1 Phenotype.
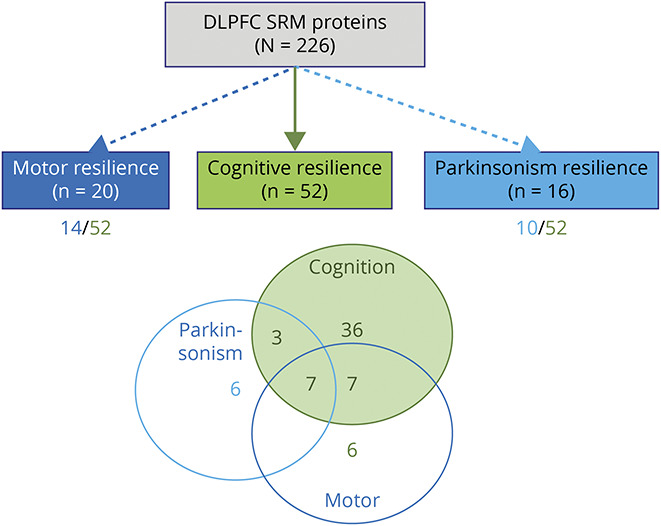
Dashed and dotted lines emerging from the prefrontal selective reactive monitoring (SRM) proteins box show results of 3 complementary analyses of the number of cortical proteins (n = 226) measured in the dorsolateral prefrontal cortex (DLPFC) related to cognitive resilience (n = 52), motor resilience (n = 20), and parkinsonism resilience (n = 16) after controlling for brain pathologies (false discovery rate <0.05). Below the boxes for motor and parkinsonism resilience is the number of proteins shared with cognitive resilience. Venn diagram shows the number of proteins shared and unique for each of the 3 phenotypes. About 70% of the proteins used to construct the cognitive resilience index (CRI) provide resilience for only cognition, and ∼30% may also provide motor resilience. A smaller number of proteins from the prefrontal cortex not included in the CRI may provide resilience for ≥1 aging motor phenotypes. These latter proteins are located outside the green overlapping cognitive and motor areas in the figure.
CRI and AD and Non-AD Brain Pathologies
ADRD Pathologies and CRI
As shown, the variance accounted for by CRI with cognitive decline is additive but reduced when combined in the same model with neuropathologies. This raises the question that some of its constituent proteins may also be related to ADRD pathologies. To test this hypothesis, we stratified participants into equal groups of having a high (above median) or a low (below median) CRI and examined the distributions and burden of 10 ADRD pathologies.
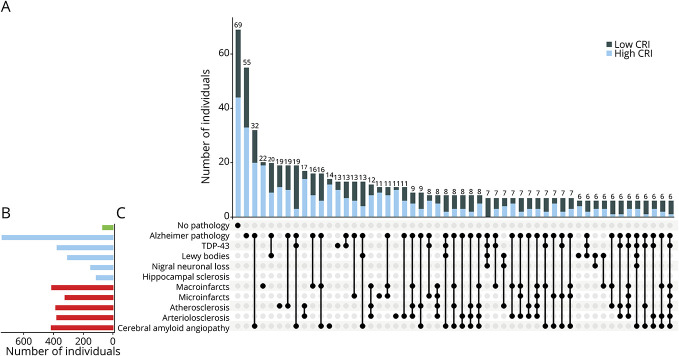
Adults with a high index had fewer ADRD pathologies (mean 2.7, SD 1.7 vs mean 3.5, SD 1.7), and fewer showed a pathologic diagnosis of AD (National Institute on Aging–Reagan) (35.7% vs 64.3%). Frequencies of common ADRD pathologies, stratified by high and low CRI, are illustrated in Figure 4. The results that individuals with a higher index had lower ADRD pathologies and less frequent neuropathologic diagnosis of AD suggest that the aggregated peptides not only may provide cognitive resilience but also may be associated with the measured level of ADRD pathologies.
Figure 4. Burden of Brain Pathologies Is Lower in Individuals With High CRI Scores.
(A) Histograms in the main panel show the frequencies of the neuropathologic indices for participants with high cognitive resilience index (CRI) (graeater than median CRI, light blue) and participants with low CRI (less than median CRI, black). Bar chart (B) shows the frequencies of mixed-brain pathologies in the analytic cohort. (C) Connected black dots on the x-axis indicate the specific combination of neuropathologies represented. (A) Histograms in the main panel show the frequencies of the neuropathologic indices for participants with high cognitive resilience index (CRI) (greater than median CRI, light blue) and participants with low CRI (less than median CRI, black). As illustrated, brain neuropathologic indices frequently co-occur. Overall, fewer combinations of different brain pathologies are observed in individuals with high CRI. TDP-43 = TAR DNA-binding protein 43.
AD Pathologic Phenotypes and Individual Peptides
To determine whether any of the 52 peptides associated with cognitive resilience are also related to ADRD pathologies, we used regression analyses to identify which of the 52 cognitive resilience proteins used to construct CRI were also associated with AD pathologic phenotypes (eTable 5, links.lww.com/WNL/B808). Nearly all of the 52 proteins aggregated into the index were also independently associated with AD pathologic phenotypes (summary AD pathology score 47 of 52 [90%], tangles 49 of 52 [94%], β-amyloid 44 of 52 [85%]). Indeed, inspection of the r2 for each of these regression models showed that these proteins accounted for 36%, 21%, and 48% of the variance of these 3 ADRD pathologies. In Figure 5, we summarize the associations between the cognitive resilience proteins and ADRD pathologic phenotypes.
Figure 5. Cortical Proteins Are Multifunctional, May Provide Resilience for Aging Phenotypes, and Are Associated With AD Pathologic Phenotypes.
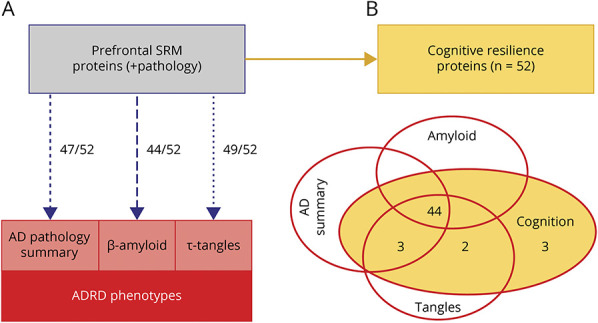
(A) Many of the cortical proteins included in the cognitive resilience index are also associated with Alzheimer disease (AD) pathologic phenotypes, including a summary score for AD pathology, β-amyloid, and tau tangles. (B) Venn diagram illustrates that most of the 52 cognitive resilience proteins are also associated with AD pathologic phenotypes. ADRD = AD and related dementia; SRM = selective reactive monitoring.
Non-AD Pathologic Phenotypes and the CRI
Because ADRDs are associated with both AD and non-AD brain pathologies, we used a logistic regression model to examine whether the CRI was also related to non-AD pathologies. We ran 9 separate models with each neuropathologic index as the outcome and CRI as the predictor. A higher index was associated with lower levels of Lewy bodies, nigral neuronal loss, TDP-43, hippocampal sclerosis, and cerebral amyloid angiopathy but not with vascular pathologic indices (eTable 6, links.lww.com/WNL/B808).
Interaction Effects of CRI With AD Pathologic Phenotypes
Our prior work suggests that some cortical proteins may modify the known association of AD pathology with cognitive decline.33 Therefore, we tested whether a higher index reduces the strength of the negative association of AD pathology with cognitive decline, i.e., is associated with a lower odds of AD dementia proximate to death. Adding interaction terms between CRI and ADRD pathology, we found that 1 SD higher on the index score reduced the detrimental effects of ADRD pathology on dementia proximate to death by 30% (OR 0.70, 95% CI 0.53–0.92, SE 0.14, p = 0.01), specifically with β-amyloid (OR 0.85, 95% CI 0.74–0.98, SE 0.07, p < 0.05) but not tangles (eTable 7, models A1 and A2, links.lww.com/WNL/B808). We repeated the analyses using MCI as an outcome, and we found similar results (eTable 7, models B1 and B2).
This interaction is illustrated in Figure 6, which compares the odds of dementia for 3 average participants with high, average, and low CRI scores.
Figure 6. Association of AD Pathology With Probability of Dementia Proximate to Death Varies With an Individual's Level of CRI.
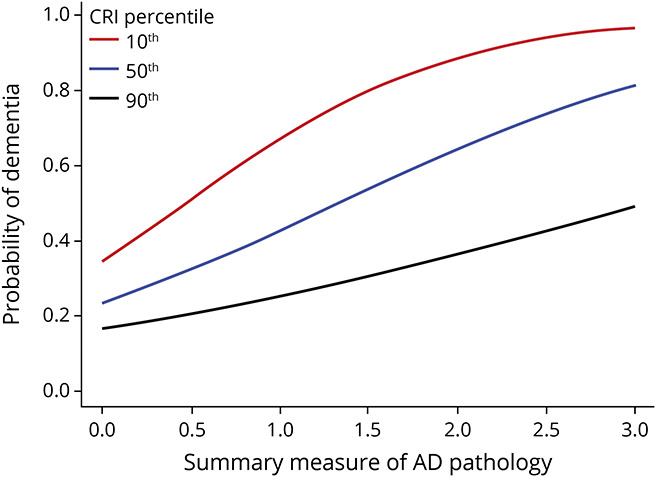
Probability of clinically diagnosed Alzheimer disease (AD) dementia proximate to death as a function of the level of AD pathology (summary measure) in participants with low (red, 10th percentile), average (blue, 50th percentile), and high (black, 90th percentile) cognitive resilience index (CRI). For all measures of AD pathology (x-axis), an individual with high CRI (black line) has a lower probability of dementia compared to an individual with low CRI (red).
Discussion
We identified and aggregated the expression levels of 52 cognitive resilience proteins measured from postmortem prefrontal cortex into a single index score in >1,000 well-characterized decedents. A higher index was associated with slower cognitive decline, lower probability of MCI and AD proximate to death, and slower rate of motor decline and progressive parkinsonism. Further analyses showed that some of the cortical proteins used to construct the index provide resilience for cognitive decline and resilience for late-life motor decline and were independently associated with ADRD pathologies. Together, these results suggest that resilience proteins may be high-value therapeutic targets for drug discovery of interventions that can offset the negative effects of ADRD pathologies via several different pathways to maintain brain health in aging adults.
While prior work has identified clinical phenotypes for resilience, ranging from behavioral and experiential measures such as education and engagement in various lifestyle activities1,8,9 to molecular mechanisms that include a number of proteins associated with cognitive resilience,5,6,14 these diverse results have been difficult to operationalize into a tool that might be used to identify adults with high or low resilience. Risk indices have been used widely to assess person-specific risk across different diseases such as coronary heart disease,34 diabetes,35 and dementia,36 by collecting diverse clinical information (e.g., vascular risk factors and diseases)37 and lifestyle measures (e.g., physical activity)38 and by aggregating multiple risk factors into an individualized predictive index to identify individuals who may benefit from personalized interventions. To leverage the rapid advances and availability of multilevel streams of genomic data requires new analytic approaches to summarize these large novel datasets into meaningful clinical tools. While our prior work has documented multiple proteins that may provide resilience, we are not aware of prior studies that aggregated diverse proteins together into person-specific risk indices of resilience. The risk index constructed in the current study can advance both research and clinical care of older adults. This index is an important first effort in translating genomic data findings into a meaningful clinical measure that provides a framework for elucidating the neurobiology of resilience. This approach would be potentially useful for risk stratification of vulnerable older adults and for monitoring the homogeneity of participants in clinical drug trials for ADRD or for assessing responses to interventions targeting neural reserve.
Although we controlled for ADRD pathologies to isolate cognitive resilience, CRI and many of its constituent proteins were also independently related to the level of AD pathology.6 In prior work, we reported that cortical resilience protein expression such as small glutamine-rich tetratricopeptide repeat-containing protein β might also have an independent association with the level of AD pathology. This study lends further support to this idea; we found that nearly all of the proteins included in the CRI were associated with AD pathologic phenotypes, including tau tangles, β-amyloid, and a summary measure of AD pathology. Together, these studies highlight that resilience proteins may have multiple functions that contribute to late-life cognitive impairment. Further mechanistic studies are needed to elucidate how resilience proteins drive cognitive decline and ADRD pathologies in aging brains.
While some cortical proteins may be related to the burden of AD pathologies, a prior report in this cohort suggests an additional mechanism; we found that higher levels of BDNF gene expression may modify the association of a given level of ADRD pathologies (β-amyloid) with the rate of cognitive decline.33 The current study extends this prior report in that we found that a higher CRI also modified the negative effect of ADRD pathologies. Together, these analyses provide evidence that cognitive resilience proteins may affect cognitive decline through at least 3 pathways. First, cognitive resilience proteins may drive cognitive decline unrelated to ADRD pathologies. Second, resilience proteins may contribute to mechanisms that affect the level of AD pathology. Third, these same proteins may also modify the negative effects of a given level of AD pathology on cognition via an interaction.
Strengths of our study include leveraging novel resources in a large numbers of older men and women, including longitudinal structured clinical assessments with validated instruments, uniform postmortem ADRD pathologies, and proteomic data analyzed under a single analytic framework to advance our understanding of the biology underlying cognitive resilience. As with other risk indices, aggregating multiple cortical proteins allowed us to summarize and apply all relevant information using 1 continuous index. The independent associations of the CRI with both cognitive and motor aging phenotypes and its validation in independent samples of older adults underscore that the CRI is a robust index that may have clinical utility not only for cognition but also for other important aging phenotypes. Additional analyses highlight that because of the complexity of its constituent proteins, the CRI may have additional functions; i.e., it is also related to the burden of ADRD pathologies and may modify the negative effects of AD pathologic phenotypes.
Our results need to be replicated in more diverse populations. While the datasets leveraged in the current study are large, the effects of individual genes and proteins may be quite small, and our results highlight the utility of aggregating multiple proteins together into a summary risk score to investigate the biology and heterogeneity of cognitive resilience.
A single brain region was chosen from which to collect the diverse streams of data analyzed in this study, so investigations of other regions of the brain are crucial to identify and quantify the effect of shared and novel region-specific proteins. While 10 ADRD pathologies were measured, other pathologies such as white matter changes39 will need to be examined in further studies. This study investigated only a limited number of proteins, so we did not conduct Gene Ontology analysis to reduce the number of proteins identified to specific molecular or functional pathways that might facilitate novel treatments. An unbiased proteome-wide study is needed to address this limitation.6
The correlations reported in this study do not allow for causal inferences and cannot be translated into clinical use without further studies. This study is best conceptualized as an initial step providing support for an approach that can be used to advance our knowledge of the biology underlying cognitive resilience. Aging brains show combinations of diverse ADRD pathologies, so treatments for an individual pathology are likely to have only a small effect on overall cognitive impairment. On the other hand, interventions targeting proteins that drive cognitive resilience could offset the effects of multiple pathologies. Yet, there are thousands of proteins in the dorsolateral prefrontal cortex that may contribute to cognitive resilience. This discovery study used our analytic approach to identify a parsimonious set of multifunctional cortical proteins that may provide cognitive resilience and resistance to ADRD pathologies. Further experiments in model organisms or human cell modeling will be crucial to characterize the causal mechanisms and pathways that link these resilience proteins with diverse AD clinical phenotypes, including cognitive or motor decline and pathologic phenotypes such as AD. This study also provides investigators therapeutic targets for further drug discovery studies to develop treatments that provide resilience even in the presence of untreatable brain pathologies. In the interim, integrative genomic studies may be able to translate our findings from decedents to living adults to catalyze the use of a CRI for risk stratification of vulnerable adults in our aging population.
Acknowledgment
The authors are deeply indebted to all participants who contributed their data and biospecimens. They are thankful to the staff of the Rush Alzheimer's Disease Center. Data used in this study are available through request via the RADC Research Resource Sharing Hub (radc.rush.edu/).
Glossary
- AD
Alzheimer disease
- ADRD
AD and related dementia
- CRI
cognitive resilience index
- FDR
false discovery rate
- MAP
Rush Memory and Aging Project
- MCI
mild cognitive impairment
- OR
odds ratio
- ROS
Religious Orders Study
- SRM
selective reactive monitoring
- TDP-43
TAR DNA-binding protein 43
Appendix. Authors
Footnotes
Editorial, page 519
Study Funding
This work was supported by NIH P30AG10161, K01AG054700, R01AG15819, R01AG17917, R01AG47976, R01AG56352, R01AG59732; U01AG46152, and U01AG61356; the Illinois Department of Public Health; and the Robert C. Borwell Endowment Fund.
Disclosure
A.R. Zammit, L. Yu, and V. Petyuk report no relevant disclosures relevant to the journal. J.A. Schneider has the following disclosures: consultant, Alnylam Pharmaceuticals, AVID Radiopharmaceuticals (subsidiary or Eli Lilly, Inc), and Takeda; and expert, National Hockey League. P.L. De Jager, H.U. Klein, D.A. Bennett, and A.S. Buchman report no relevant disclosures relevant to the journal. Go to Neurology.org/N for full disclosures.
References
- 1.Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8(3):448-460. [PubMed] [Google Scholar]
- 3.Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol. 2012;11(11):1006-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katzman R, Terry R, DeTeresa R, et al. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988;23(2):138-144. [DOI] [PubMed] [Google Scholar]
- 5.Yu L, Petyuk VA, Gaiteri C, et al. Targeted brain proteomics uncover multiple pathways to Alzheimer's dementia. Ann Neurol. 2018;84(1):78-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu L, Tasaki S, Schneider JA, et al. Cortical proteins associated with cognitive resilience in community-dwelling older persons. JAMA Psychiatry. 2020;77(11):1172-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, et al. Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 2020;16(9):1305-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyle PA, Buchman AS, Wilson RS, Yu L, Schneider JA, Bennett DA. Effect of purpose in life on the relation between Alzheimer disease pathologic changes on cognitive function in advanced age. Arch Gen Psychiatry. 2012;69(5):499-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scarmeas N, Stern Y. Cognitive reserve and lifestyle. J Clin Exp Neuropsychol. 2003;25(5):625-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stern Y. The concept of cognitive reserve: a catalyst for research. J Clin Exp Neuropsychol. 2003;25(5):589-593. [DOI] [PubMed] [Google Scholar]
- 11.Grafton ST, Volz LJ. From ideas to action: the prefrontal-premotor connections that shape motor behavior. Handb Clin Neurol. 2019;163:237-255. [DOI] [PubMed] [Google Scholar]
- 12.Bennett DA. Mixed pathologies and neural reserve: implications of complexity for Alzheimer disease drug discovery. PLoS Med. 2017;14(3):e1002256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mostafavi S, Gaiteri C, Sullivan SE, et al. A molecular network of the aging human brain provides insights into the pathology and cognitive decline of Alzheimer's disease. Nat Neurosci. 2018;21(6):811-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramos-Miguel A, Jones AA, Petyuk VA, et al. Proteomic identification of select protein variants of the SNARE interactome associated with cognitive reserve in a large community sample. Acta Neuropathol. 2021;141(5):755-770. [DOI] [PubMed] [Google Scholar]
- 15.Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the religious orders study. Curr Alzheimer Res. 2012;9(6):628-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the Rush Memory and Aging Project. Curr Alzheimer Res. 2012;9(6):646-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson RS, Boyle PA, Yu L, Segawa E, Sytsma J, Bennett DA. Conscientiousness, dementia related pathology, and trajectories of cognitive aging. Psychol Aging. 2015;30(1):74-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59(2):198-205. [DOI] [PubMed] [Google Scholar]
- 19.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197-2204. [DOI] [PubMed] [Google Scholar]
- 20.Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA. Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis. 2018;64(s1):S161–S189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buchman AS, Shulman JM, Nag S, et al. Nigral pathology and parkinsonian signs in elders without Parkinson disease. Ann Neurol. 2012;71(2):258-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA. The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis. 2009;18(3):691-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider JA, Arvanitakis Z, Yu L, Boyle PA, Leurgans SE, Bennett DA. Cognitive impairment, decline and fluctuations in older community-dwelling subjects with Lewy bodies. Brain. 2012;135(pt 10):3005-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66(2):200-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyle PA, Yu L, Wilson RS, Leurgans SE, Schneider JA, Bennett DA. Person-specific contribution of neuropathologies to cognitive loss in old age. Ann Neurol. 2018;83(1):74-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167-202. [DOI] [PubMed] [Google Scholar]
- 27.Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1(1):59-65. [DOI] [PubMed] [Google Scholar]
- 28.Funahashi S, Andreau JM. Prefrontal cortex and neural mechanisms of executive function. J Physiol Paris. 2013;107(6):471-482. [DOI] [PubMed] [Google Scholar]
- 29.Preuss TM, Wise SP. Evolution of prefrontal cortex. Neuropsychopharmacology. 2022;47(1):3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindberg O, Westman E, Karlsson S, et al. Is the subcallosal medial prefrontal cortex a common site of atrophy in Alzheimer's disease and frontotemporal lobar degeneration? Front Aging Neurosci. 2012;4:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rush Alzheimer's Disease Center Research Resource Sharing Hub. Accessed September, 09, 2021. radc.rush.edu. [Google Scholar]
- 32.Buchman AS, Leurgans SE, Boyle PA, Schneider JA, Arnold SE, Bennett DA. Combinations of motor measures more strongly predict adverse health outcomes in old age: the Rush Memory and Aging Project, a community-based cohort study. BMC Med. 2011;9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buchman AS, Yu L, Boyle PA, Schneider JA, De Jager PL, Bennett DA. Higher brain BDNF gene expression is associated with slower cognitive decline in older adults. Neurology. 2016;86(8):735-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D'Agostino RB Sr, Grundy S, Sullivan LM, Wilson P; CHD Risk Prediction Group. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286(2):180-187. [DOI] [PubMed] [Google Scholar]
- 35.Lindström J, Tuomilehto J. The Diabetes Risk Score: a practical tool to predict type 2 diabetes risk. Diabetes Care. 2003;26(3):725-731. [DOI] [PubMed] [Google Scholar]
- 36.Sindi S, Calov E, Fokkens J, et al. The CAIDE Dementia Risk Score App: the development of an evidence-based mobile application to predict the risk of dementia. Alzheimers Dement (Amst). 2015;1(3):328-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22(3):312-318. [DOI] [PubMed] [Google Scholar]
- 38.Chiuve SE, Cook NR, Shay CM, et al. Lifestyle-based prediction model for the prevention of CVD: the Healthy Heart Score. J Am Heart Assoc. 2014;3(6):e000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim N, Yu L, Dawe R, et al. Microstructural changes in the brain mediate the association of AK4, IGFBP5, HSPB2, and ITPK1 with cognitive decline. Neurobiol Aging. 2019;84:17-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data included in these analyses are available the Rush Alzheimer's Disease Center Research Resource Sharing Hub.31 Descriptions of the studies and data can be found in this hub. Qualified investigators may create an account and submit requests for deidentified data.