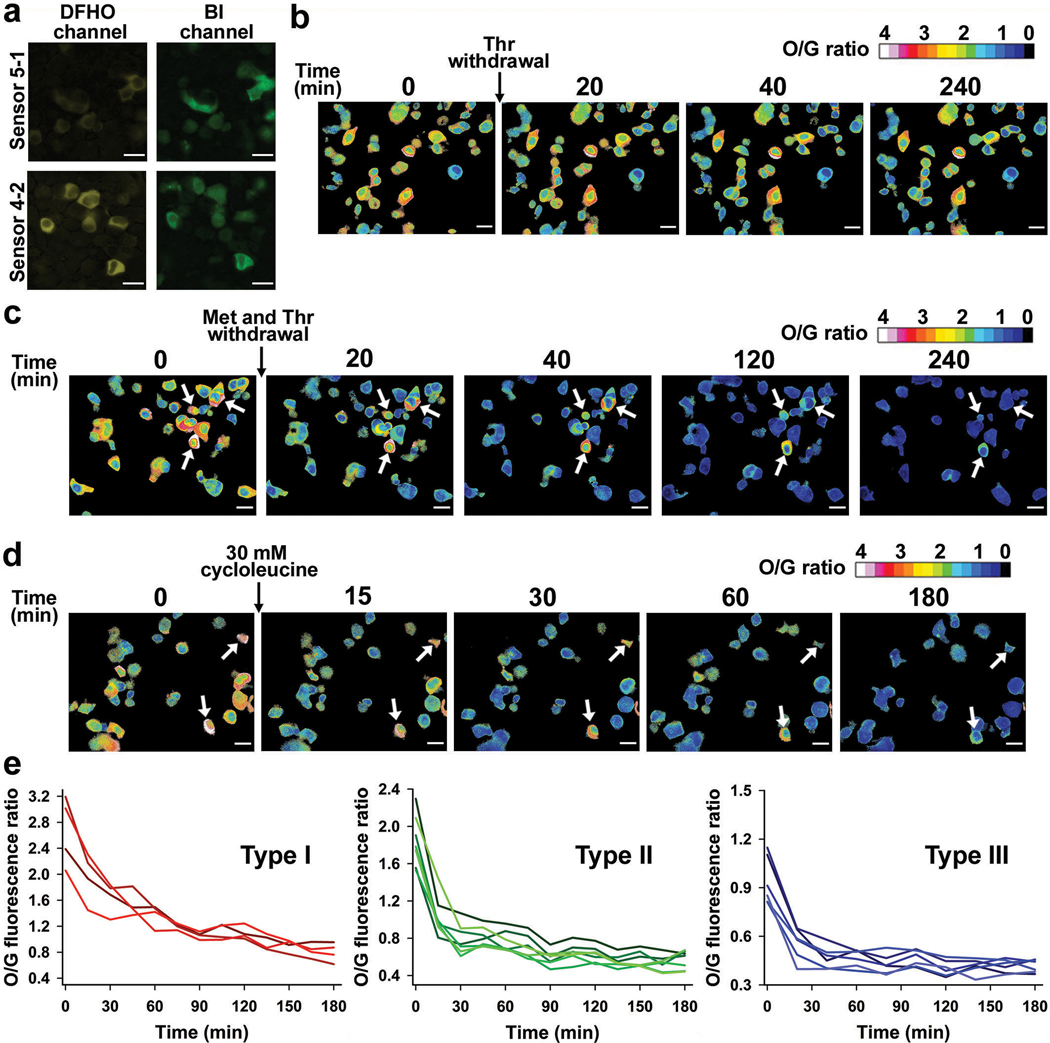
Extended Data Fig. 10. Effect of different amino acids depletion and cycloleucine treatment on SAM level in HCT116 cells.
a, Both Squash-SAM sensors 4–2 and 5–1 produced signal that is substantially higher than the background in both the channels. We used 5–1 because it has higher dynamic range. Scale bar, 20 μm.
b, Threonine depletion does not change SAM levels in mESCs (see Fig. 5a). To test if this also holds in HCT116 cells, we expressed Squash-SAM sensor 5–1 in HCT116 cells and monitored SAM level after threonine depletion. Most cells did not show any notable change in SAM levels, consistent with a lack of functional threonine dehydrogenase enzyme in these cells.
c, Although threonine depletion does not change SAM levels (panel b), threonine could be important in methionine-depleted cells. To test this, we expressed Squash-SAM sensor 5–1 in HCT116 cells and monitored SAM levels for 240 min after depleting both threonine and methionine. Threonine depletion did not exacerbate the drop in SAM levels, suggesting that threonine does not have a major role in SAM levels in these cells.
d, Circular Squash-SAM sensor 5–1 expressed in HCT116 cells showed marked drop in SAM after cycloleucine (30 mM final) treatment, indicating MAT2A is required for SAM biosynthesis in these cells. A rapid drop in SAM levels was observed in 30 min for most cells. The cells indicated by white arrow (in c and d) start with a very high level of SAM and show slower drop in SAM level over time compared to other cells.
e, HCT116 cells have three different cell populations based on baseline SAM levels (see Fig. 5d and 5e). We quantified the average cellular ratio of O/G fluorescence for these cells at 15 min intervals after cycloleucine (30 mM final) treatment. All three populations showed slightly faster drop in SAM levels with cycloleucine treatment compared to depletion of methionine and threonine together.