Extended Data Fig. 6: Deep mutational scanning of the ammocidin binding site on ATP Synthase.
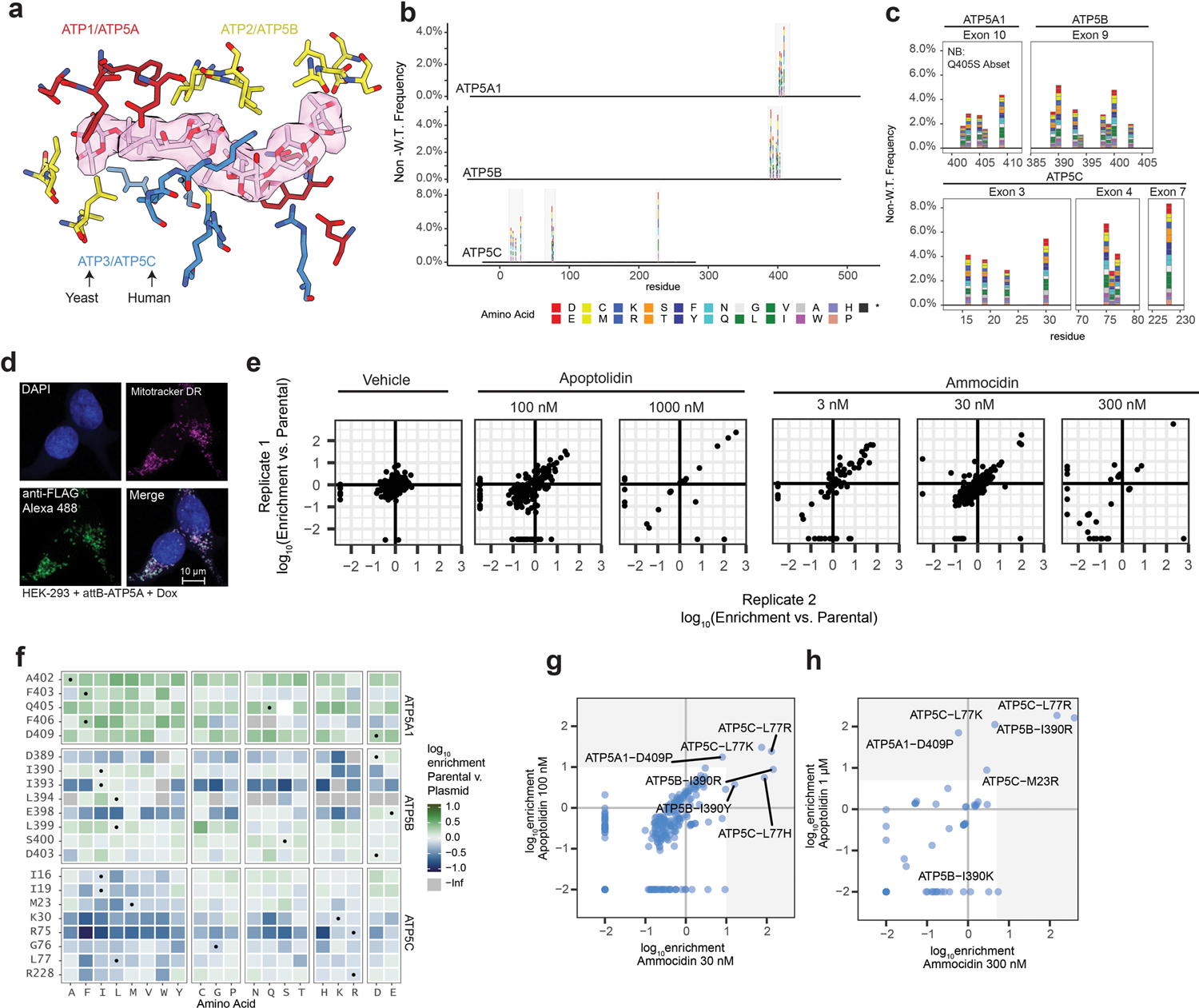
a, Selection of residues for mutagenesis on α (Yeast ATP1, Human ATP5A – red), β (Yeast ATP2, Human ATP5B – yellow), and γ (Yeast ATP3, Human ATP5C – blue) subunits within 4.5 Å of ammocidin or apoptolidin; b, c, Validation of ATP synthase variant library after nicking mutagenesis showing the frequency of non-wildtype variants at each position, with residue 1 corresponding to the first residue of the mature peptide; d, Assessment of reproducibility between biological replicates showing consistent enrichment of resistance mutations, variants which were not observed were set to −2.5; e, Confirmation of proper mitochondrial localization of the landing-pad expressed ATP5A by immunofluorescence at 63x in HEK-293T landing pad cells; f, Comparison of variant frequencies observed after selection for successful integration compared to the original library, variants which were observed in the original library but not detected after integration were set to -Inf. Variants which exhibit negative enrichment or complete loss are thought to exhibit decreased fitness relative to the WT alleles; g, Comparison of variants enriched by apoptolidin and ammocidin at medium dose (100 nM / 30 nM) or h, high (1 μM / 300 nM) demonstrating that apoptolidin and ammocidin select for similar resistance mutations.
