FIGURE 5.
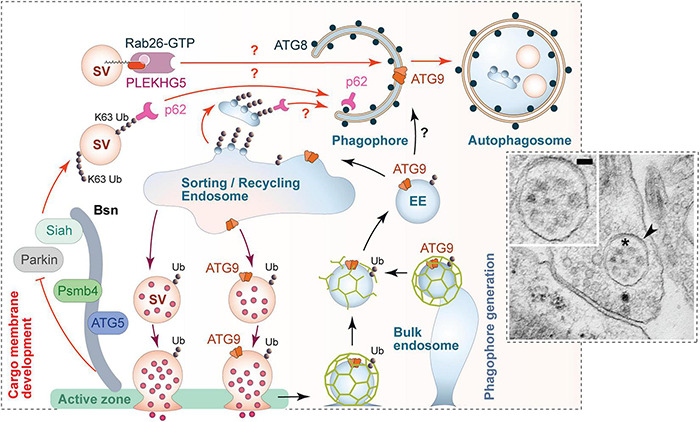
Scenario for the regulation of presynaptic autophagy. Autophagy within presynaptic boutons appears to be locally regulated and mediated via the convergence of two major facets of autophagy. (1) Local tagging of aged and/or damaged proteins/organelles by the ubiquitination system. The active zone protein Bassoon is one regulator of this process by scaffolding E3 ligases such as Siah1 and Parkin. Bassoon can also control the induction of phagophore formation by inhibiting the activity of ATG5 and proteasome function via its binding to Psmb4 proteasomal subunit. (2) The formation of phagophore membranes, which requires an interplay between numerous proteins essential for the regulated conjugation of ATG8/LC3 to membranes containing the integral membrane protein ATG9. Many of these proteins are part of the hidden proteome of SVs (see Figure 3) and thus available for the rapid production of these membranes. This aspect of autophagy seems to be coupled to synaptic activity and the sorting and recycling of SV proteins through early endosomes. While not well understood, this compartment is well positioned to not only sort healthy ensembles of proteins regenerating functional SVs, but also damaged ubiquitinated proteins for engulfment into newly forming phagophores. This latter step requires the small GTPase Rab26 and its guanine exchange factor PLEKHG5, as well as autophagy adaptor proteins, like p62/SQSTM1, which binds both poly-ubiquitin chains and ATG8s. The inserted electron micrograph demonstrates the uptake of entire SV-like structures (asterisk) into autophagic vacuoles (arrowhead) within a presynapse (taken from Hoffmann-Conaway et al., 2020; size bar, 50 nm). Finally, the sorting/recycling endosomes also appears to function in the regeneration of SV-like membranes that carry ATG9, providing autophagic support for boutons in subsequent rounds of neurotransmitter release.
