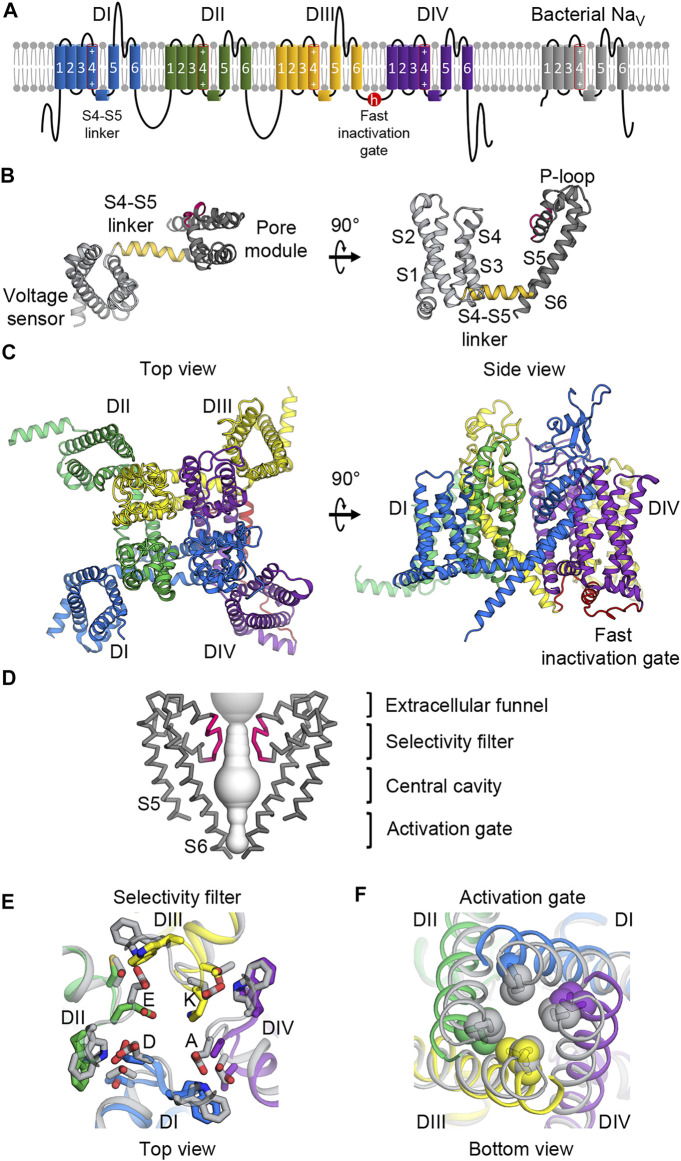
FIGURE 1.
Molecular architecture of NaV channels. (A) Topology diagram of mammalian and bacterial NaV channels in the lipid membrane. Left, mammalian NaV channels comprise four domains (DI—blue; DII—green; DIII—yellow; DIV—purple) with the fast inactivation gate (red) in the DIII-DIV loop. Right, bacterial NaV channels contain one domain and lacks the fast inactivation gate. The S4 segments include an array of positive gating charge arginines. (B) Structure of the core NaV channel domain from bacterial NaVAb. The 6-TM domain consists of the voltage sensing domain (S1 to S4, light gray) and the pore module (S4 to S5, dark gray) connected by the S4-S5 linker (gold). Between the S5 and S6 segments is the P-loop including the selectivity filter (pink). (C) Structure of the mammalian NaV α-subunit. Four domains colored as in (A) form a domain-swapped tertiary structure with the pore modules lining the pore architecture at the center, and the VSDs in the periphery.(D) Molecular architecture of the pore. The pore modules form a sodium permeable tunnel (light gray volume). For clarity, only two in-plane domains are illustrated. From top to bottom: the extracellular funnel attracts and concentrates Na+ ions; the selectivity filter (pink) selects for passages of hydrated Na+ ions; the central cavity contains hydrophobic inner surface; the activation gate formed by the S6 segments provides an iris-like exit. (E) Extracellular view of the selectivity filter superimposed between mammalian and bacterial NaV channels. Mammalian NaV channels colored as in (A) contain the DEKA motif to which each domain contributes an amino acid while the bacterial selectivity filter (gray) is symmetric with glutamate residues forming a high-field-strength site. (F) Intracellular view of the activation gate superimposed between mammalian and bacterial NaV channels. The S6 segments interlace with hydrophobic residues sealing the exit when the channel is closed, and dilating when the channel is open.