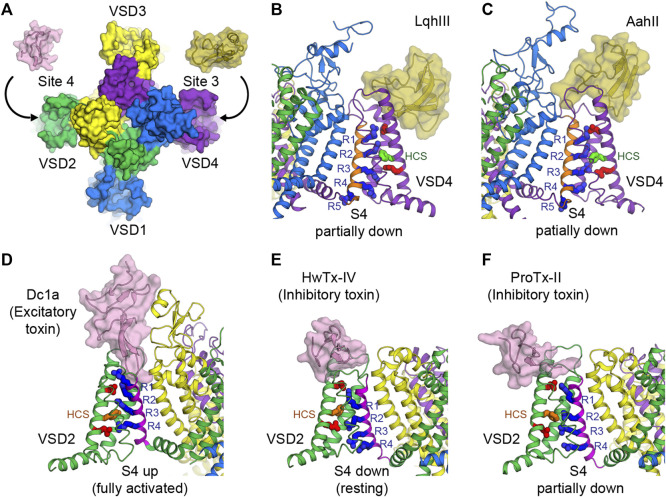
FIGURE 4.
Toxins that bind to voltage sensors to modulate the pore. (A) Receptor sites 3 and 4 at the extracellular aqueous clefts of VSD4 and VSD2, respectively, are targets of animal cysteine knot peptides. (B,C) Site 3 scorpion α-toxins LqhIII (B) and AahII (C) bind to VSD4 of rat NaV1.5 and cockroach NaVPas/human NaV1.7-VSD4 chimera, respectively, to stabilize S4 (orange) in the partially down conformation, as 2 gating charges are external to the HCS. The gating charge arginines (R1–R5) are shown in blue. Extracellular and intracellular negative clusters are shown in red. Phenylalanine in the HCS is shown in green. (D) Site 4 excitatory spider toxin Dc1a binds to VSD2 of cockroach NavPas and traps S4 (magenta) in the activated state, as three gating charges are external to the HCS. Phenylalanine in the hydrophobic constriction site (HCS) is shown in orange. (E,F) Site 4 inhibitory spider toxins HwTx-IV (E) and ProTx-II (F) trap VSD2 of NaVAb/human NaV1.7-VSD2 chimera in the resting state and the deactivated state, as 1 and 1.5 gating charges are external to the HCS, respectively.