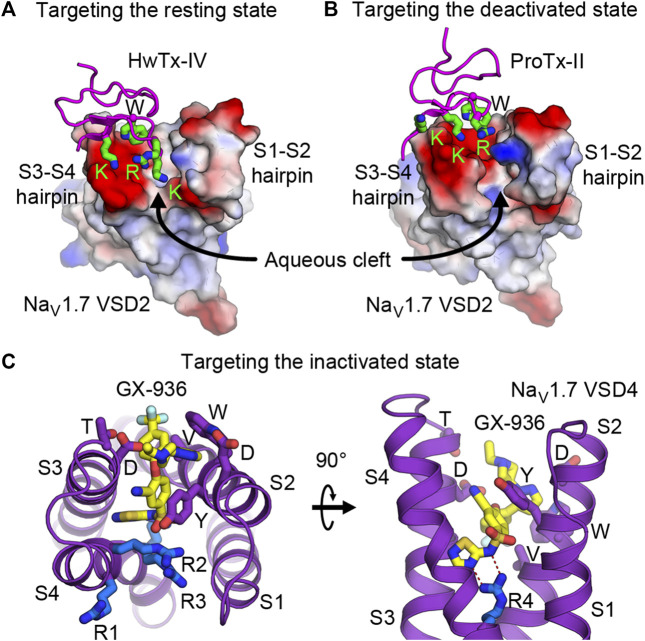
FIGURE 5.
Druggability of the voltage sensor in the resting, deactivated, and inactivated states. (A,B) Structures of HwTx-IV (A) and ProTx-II (B) bound to the aqueous cleft of NaVAb/human NaV1.7-VSD2 chimera. Molecular surface of VSD2 is colored by electrostatic potentials (red—negative; blue—positive). Positively charged lysine and arginine residues of the toxins interact with negatively charged residues located in the S3-S4 hairpin. A tryptophan residue from the toxins provides additional van der Waals interaction with the receptor. (C) GX-936 aryl sulfonamide antagonist bound to the aqueous cleft of NaVAb/human NaV1.7-VSD4 chimera. Carbon atoms of GX-936 are colored in yellow. Residues from VSD4 forming the binding site are shown as sticks. The gating charge arginines R1-R4 are shown in blue.