Table 10.
SAR at the pyridinone N1-aryl ring.
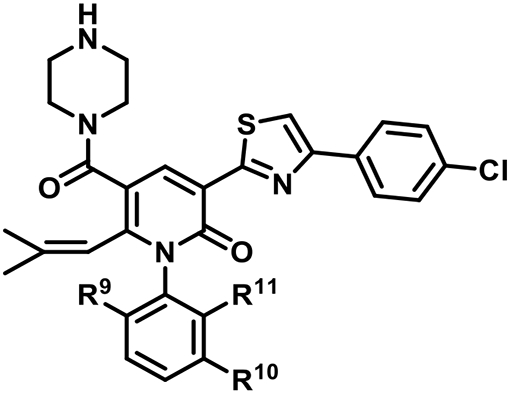
| ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Biochemical | IDH1 R132H | IDH1 R132C | ||||
|
|
||||||
| Compd | R9 | R10 | R11 | IC50(μM) ± SDa | IC50(μM)± SDa | |
| 92 | Et | H | Et | 0.044 ± 0.003 | 0.058 ± 0.010 | |
| 113 | H | H | H | 0.33 ± 0.00 | 3.2 ± 0.6 | |
| 114 | Et | H | H | 0.056 ± 0.004 | 0.16 ± 0.01 | |
| 115 | Et | H | Me | 0.27 ± 0.00 | 2.2 ± 0.2 | |
| 116 | Et | H | Cl | 0.044 ± 0.003 | 0.055 ± 0.004 | |
| 117 | Et | Me | H | 0.044 ± 0.003 | 0.11 ± 0.02 | |
|
|
||||||
| 118 | 0.114 ± 0.007 | 0.075 ± 0.000 | ||||
| (+)−118 | 0.12 ± 0.02 | 0.13 ± 0.03 | ||||
| (−)−118 | Et | OMe | H | 0.42 ± 0.05 | 0.67 ± 0.04 | |
|
|
||||||
| 119 | 0.114 ± 0.007 | 0.10 ± 0.01 | ||||
| (+)−119 | 0.081± 0.005 | 0.072 ± 0.005 | NCATS-SM5637, NSC 791985 | |||
| (−)−119 | Et | Cl | H | 0.25 ± 0.04 | 0.54 ± 0.10 | |
|
|
||||||
| AG-120 | 0.065 ± 0.009 | 0.063 ± 0.015 | ||||
IC50 values were determined utilizing the diaphorase and resazurin-coupled R132H and R132C mIDH1 assays. All compounds showed >80% inhibition at 38 μM, the highest concentration tested.
