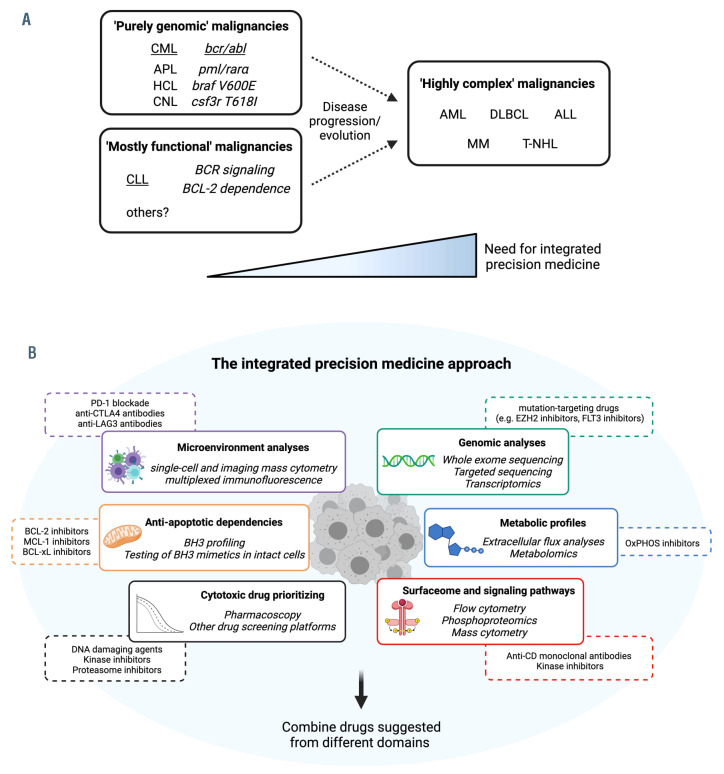
Figure 5.
Integrated precision medicine for ‘highly complex’ hematologic malignancies. (A) Classification of hematologic malignancies based on their molecular/functional complexity and treatment requirements. In chronic myeloid leukemia, the prototype of ‘purely genomic’ malignancies, a single genomic aberration drives disease biology and treatment modalities. In chronic lymphocytic leukemia, a model for ‘mostly functional’ malignancies, a few functional pathways (Bcell receptor signaling, BCL-2 dependence) sustain leukemic growth and have proven to be the best drug targets so far. In acute myeloid leukemia and other ‘highly complex’ malignancies, multiple driver mutations and functional oncogenic pathways co-occur to promote cancer growth and escape treatments. This category might be approached with an integrated precision medicine strategy. (B) Integrated precision medicine is based on interrogation of different static (i.e., microenvironment, surfaceome, genomics) and functional (i.e., anti-apoptotic and metabolic dependencies, signaling pathways, drug sensitivity) domains through dedicated assays (italics). Each of these assays will provide information about different tumor-specific vulnerabilities (e.g., BH3 profiling might highlight BCL-2 dependence, extracellular flux analysis might indicate oxidative phosphorylation utilization, microenvironment analysis might demonstrate CTLA-4-based interactions). Eventually, a combination of drugs targeting vulnerabilities from different domains is suggested, with potential benefits against ‘highly complex’ malignancies. CML: chronic myeloid leukemia; APL: acute promyelocytic leukemia; HCL: hairy cell leukemia; CNL: chronic neutrophilic leukemia; AML: acute myeloid leukemia; DLBCL: diffuse large B-cell lymphoma; ALL: acute lymphoblastic leukemia; MM: multiple myeloma; T-NHL: T-cell non-Hodgkin lymphoma; OxPHOS: oxidative phosphorylation.