Fig. 3 |. Endogenous sources of dsRNA and cellular regulatory processes.
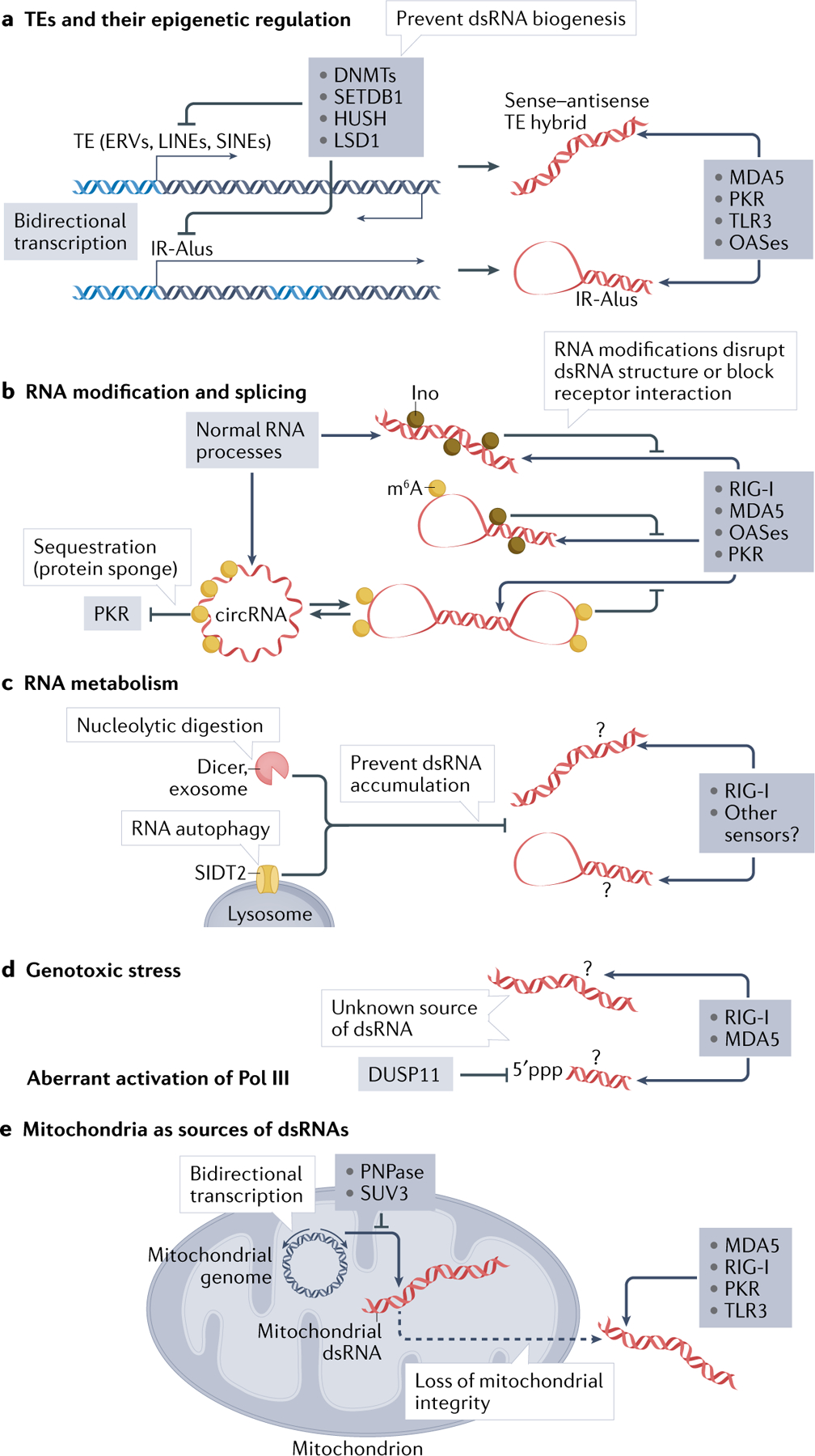
Cells have diverse endogenous sources of double-stranded RNA (dsRNA) and utilize multiple mechanisms to suppress its biogenesis and accumulation. a | Epigenetic derepression of transposable elements (TEs), such as endogenous retroviruses (ERVs), long interspersed nuclear elements (LINEs) and short interspersed nuclear elements (SINEs), can lead to dsRNA generation. These elements can be transcribed in a bidirectional manner or as an inverted repeat, forming sense–antisense hybrid or fold-back hairpin dsRNA. Biogenesis of TE-based dsRNAs is normally suppressed by epigenetic silencing mechanisms involving DNA methyltransferases (DNMTs), the histone H3 K9 methyltransferase SETDB1, its partner the human silencing hub (HUSH) and the histone demethylase LSD1. The only exception is inverted repeat Alus (IR-Alus), some of which are constitutively produced within the 3′ untranslated region of many mRNAs. b | Once transcribed, cellular RNAs are regulated by post-transcriptional modifications, such as adenosine deamination to inosine (Ino) and N6-adenosine methylation (to produce N6-methyladenosine (m6A)), both of which disrupt the structure of dsRNA and lower its immunogenicity (shown by inhibitory arrows). Deregulation of these (and potentially other) RNA modifications can result in the recognition of normal cellular transcripts as foreign, owing to the formation of local duplex structures. Splicing inhibition can lead to an increase in the levels of dsRNAs, as a result of the increase in the levels of transcripts with retained introns, which may form double-stranded structures. Splicing is also associated with the generation of circular RNAs (circRNAs), which can form dsRNA structures more easily than their linear counterparts. On the one hand, these dsRNA structures can be recognized by RIG-I, but this is negatively regulated by m6A modification, normally present in circRNAs. On the other hand, circRNAs can also act as protein sponges and sequester protein kinase R (PKR) and prevent its activation in sterile conditions. c | RNA degradation mechanisms, such as those involving Dicer, RNA exosome complex and the lysosomal RNA transporter SIDT2, may prevent excessive accumulation of dsRNA through poorly understood mechanisms (question marks). d | Genotoxic stress (for example, resulting from exposure to ionizing radiation) and aberrant activation of RNA polymerase III (Pol III; for example when MYC is activated in cancer) can promote biogenesis of RLR-stimulatory dsRNAs, but the precise nature of these dsRNAs remains unclear (question marks). These may be aberrantly processed RNA components of the spliceosome (U1 and U2 small nuclear RNAs) or products of Pol III which contain 5′-triphosphate (5′ppp). The recognition of the latter could be regulated by the phosphatase DUSP11, which can remove 5′ppp. e | Mitochondria are a rich source of dsRNA as mitochondrial RNAs are produced by bidirectional transcription of the circular DNA. Normally, the level of mitochondrial dsRNA is regulated by the mitochondrial RNA degradosome, which includes the nuclease polynucleotide phosphorylase (PNPase) and the helicase SUV3. During mitochondrial dysregulation, mitochondrial dsRNA can gain access to cytosolic dsRNA sensors and activate them through a poorly understood mechanism. OAS, oligoadenylate synthase; TLR3, Toll-like receptor 3.
