Fig. 4 |. Consequences of dsRNA recognition.
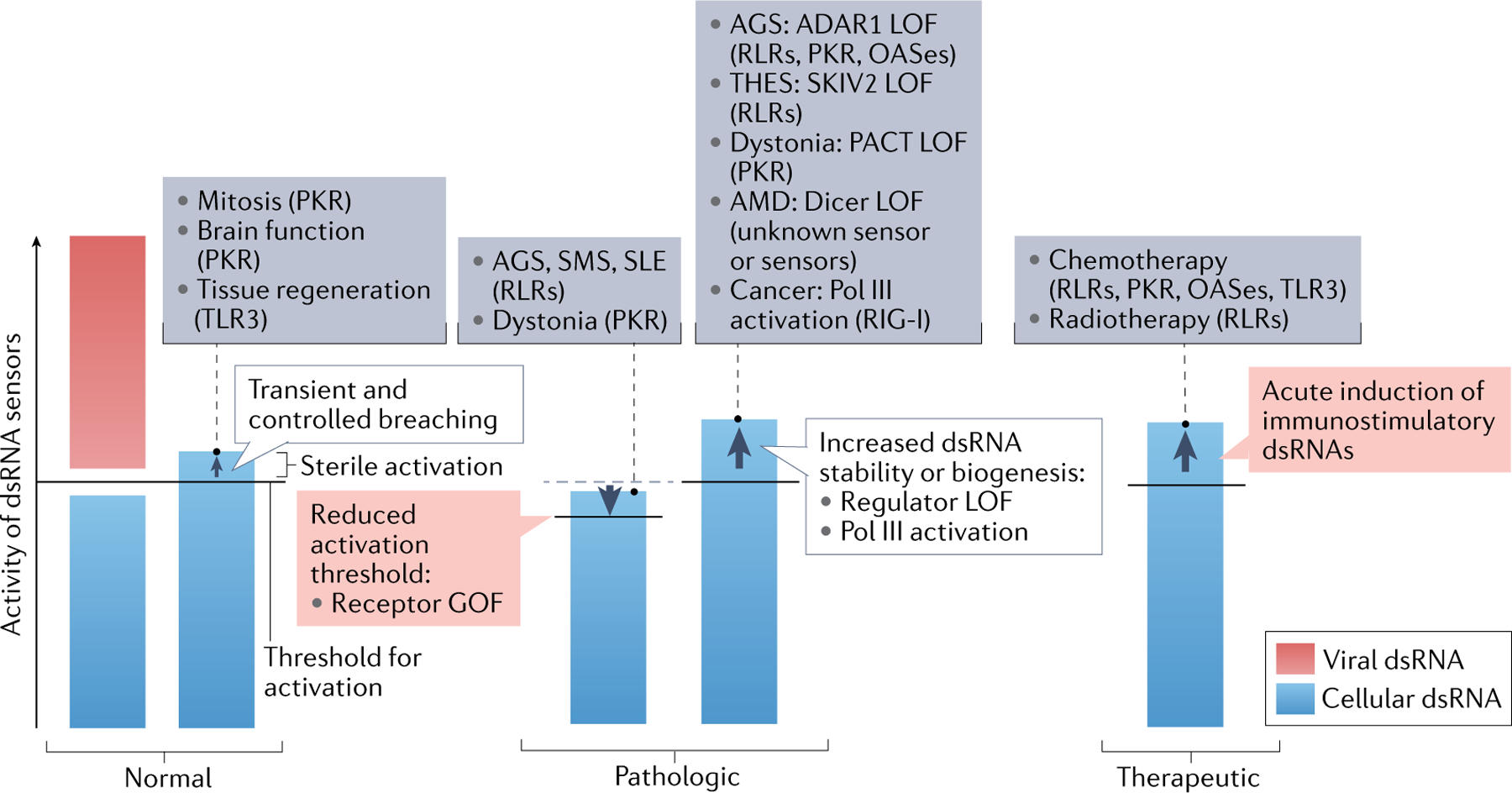
Sterile activation of double-stranded RNA (dsRNA) sensors can occur in normal, pathologic and therapeutic conditions. Here, we use an immunological threshold model to summarize examples in each category. dsRNA sensors have an evolutionarily optimized activation threshold that allows the receptors to tolerate a certain level and certain kinds of dsRNAs (for example, dsRNA with short and imperfect complementarity is normally tolerated by MDA5), while those RNAs beyond the threshold (for example, viral dsRNA) would activate the dsRNA sensors. In normal conditions, the levels of cellular dsRNAs are well below the activation threshold of dsRNA sensors. However, there are cases where a subset of cellular dsRNAs breach the threshold in a transient and controlled fashion, and innate immune functions of the dsRNA sensors are integrated into the normal biological processes. This includes protein kinase R (PKR) activation during mitosis, neuronal excitation in the brain and Toll-like receptor 3 (TLR3) activation during tissue regeneration in the skin. By contrast, constitutive and uncontrolled breaching of the tolerance threshold leads to pathogenesis of immune disorders and other diseases. This can occur through gain-of-function (GOF) mutations of the dsRNA sensor, which lowers the threshold, leading to misrecognition of otherwise inert cellular dsRNA. Such cases include Aicardi–Goutières syndrome (AGS), Singleton–Merten syndrome (SMS) and systemic lupus erythematosus (SLE) caused by GOF RIG-I-like receptors (RLRs) and dystonia caused by GOF PKR. Alternatively, similar diseases can be caused by loss-of-function (LOF) mutations in the regulators. For example, LOF in ADAR1 causes AGS through constitutive activation of RLRs, PKR or oligoadenylate synthases (OASes), LOF in SKIV2 causes trichohepatoenteric syndrome (THES), where constitutive activation of RIG-I is thought to contribute, LOF in PACT causes dystonia through constitutive activation of PKR and LOF in Dicer has been associated with age-related macular degeneration (AMD), although in the last case the exact sensor involved has not been determined. In addition, increased generation of immunostimulatory dsRNAs has been observed in cancer, where aberrant activation of RNA polymerase III (Pol III) downstream of the activity of the oncogenic protein MYC leads to increased generation of RNAs prone to forming secondary structures and RNAs containing 5′-triphosphate, which are recognized by RIG-I. Finally, immune functions of dsRNA sensors can be leveraged in cancer immunotherapy. Chemotherapy and radiotherapy were shown to confer anticancer efficacy partly by inducing biogenesis of immunostimulatory dsRNAs, which activate a broad range of dsRNA sensors.
