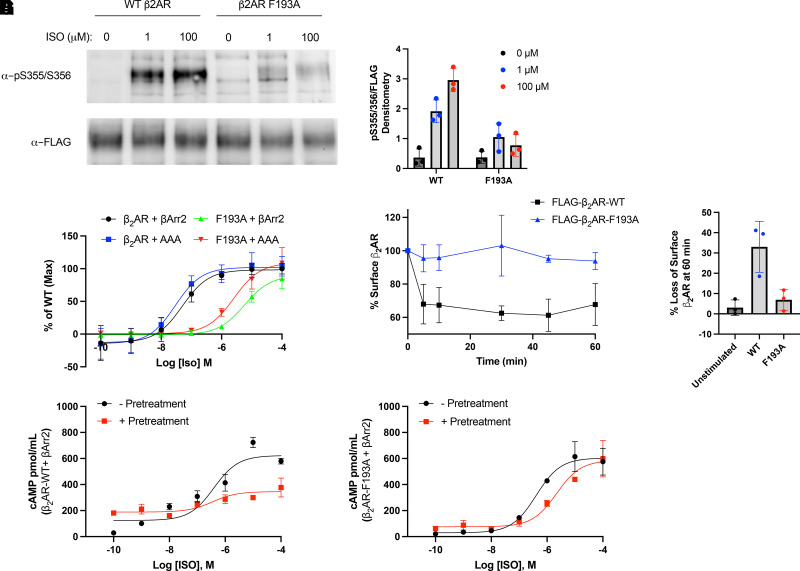
Fig. 3.
G protein-coupled receptor kinase phosphorylation, receptor internalization and desensitization for wild-type (WT) and mutant β2AR. (A) Representative blot for agonist promoted phosphorylation of the β2-adrenergic receptor (β2AR). Human embryonic kidney 293 (HEK 293) cells expressing FLAG-β2AR-WT or FLAG-β2AR-F193A were stimulated with 0, 1, or 100 μM of isoproterenol (ISO) for 10 minutes. Cells were lysed, and the FLAG-β2AR was immunoprecipitated. Phosphorylation at serines 355 and 356 was analyzed by western blot using a pSer355/356 antibody, while total β2AR was measured using an anti-FLAG antibody. The western blot is representative of at least 3 independent experiments and is quantified in panel (B). (C) ISO dose response curve for WT or AAA β-arrestin binding to β2AR wild-type and β2AR-F193A as measured by bioluminescence resonance energy transfer. HEK 293 cells co-transfected with β-arrestin2-GFP10 or β-arrestin2-AAA-GFP10 and indicated β2AR-RlucII WT or β2AR-F193A-RlucII constructs were incubated with coelenterazine 400a for 2 minutes and then stimulated with indicated concentrations of ISO. Data for dose response curve was taken 12 minutes post ISO addition. n = 3 independent experiments. (D) Effect of F193A mutation on agonist-promoted internalization of the β2AR. HEK 293 cells expressing FLAG-β2AR-WT or FLAG-β2AR-F193A were stimulated with 1 μM of ISO for up to 60 minutes. Cells were fixed, and receptor surface expression was measured by ELISA. Loss of cell surface expression at 60 minutes is quantified in (E). n = 3 independent experiments. HEK 293 cells expressing β2AR-WT (F) or β2AR-F193A (G) were desensitized by incubating with 1 μM of ISO, washing with PBS, and then re-stimulating with ISO at the indicated concentrations for 10 minutes. Cells were lysed, and cAMP production was measured by ELISA. n = 3 independent experiments.