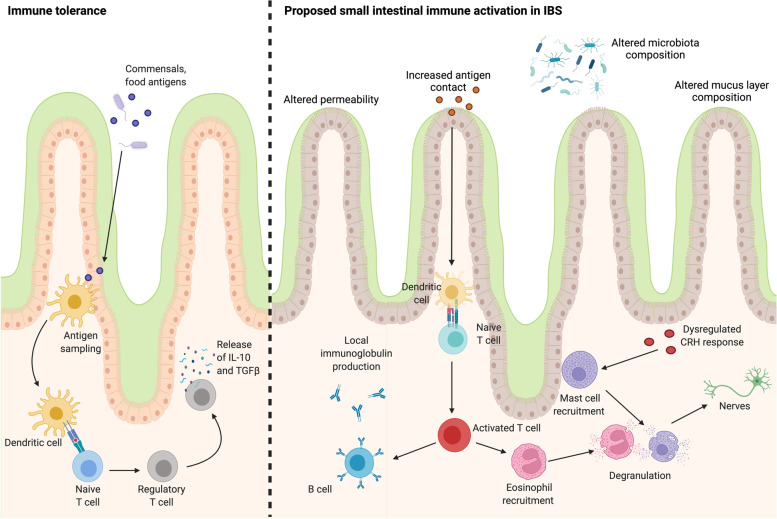
Fig. 2.
Hypothesised immune mechanisms potentially involved in small intestinal dysfunction in IBS. The small intestinal immune system actively modulates tolerance to commensal microbes and food components to maintain homeostasis, in conjunction with the mucosal barrier and mucus layer. In this process, antigens are sampled by dendritic cells and presented to naïve T cells. The differentiation of these cells to regulatory T cells results in the release of interleukin-10 and transforming growth factor beta, which actively suppresses inflammatory immune responses. In contrast, physiological abnormalities in the composition of the mucus layer, coupled with altered mucosal permeability and changed microbial community composition in IBS patients may allow for increased antigen contact with the mucosa and a dysregulated or increased stress response. In this environment, antigen presentation may result in the activation of T cell subsets that drive B cell maturation and specific antibody production that is likely localised to the gastrointestinal tract. The activation of the adaptive immune system may drive the recruitment of eosinophils and mast cells, which degranulate and release inflammatory mediators. The release of these mediators near enteric nerves is likely to promote abnormal signalling and may result in visceral pain. Altered or enhanced stress signalling may also enhance this eosinophil and mast cell response to further contribute to immune activation. However, these pathways require further investigation in IBS patients compared to controls to demonstrate the mechanisms underlying intestinal immune activation. The image was created using BioRender.com