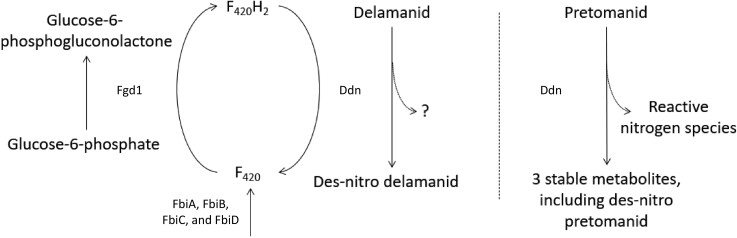
Figure 1.
Schematic overview of the metabolic activation of delamanid and pretomanid by mycobacteria, adapted with permission from Liu et al.23 and Rifat et al.36 Delamanid and pretomanid are prodrugs that require activation by deazaflavin (F420)-dependent nitroreductase (Ddn). Redox cycling of deazaflavin cofactor 420, or F420, is crucial in this process, which is mediated by glucose-6-phosphate dehydrogenase (Fgd1)12,23,35,132,133 and Ddn.10,26–28 Synthesis of F420 depends on FbiA, FbiB, FbiC and FbiD.12,36–38,134 Bio-activation of delamanid by Ddn results in the formation of inactive des-nitro-imidazooxazole.10,135 The active intermediate for delamanid has not yet been identified. Activation of pretomanid, on the other hand, generates three stable, inactive metabolites, as well as reactive nitrogen species which are responsible for respiratory poisoning by pretomanid.26,28