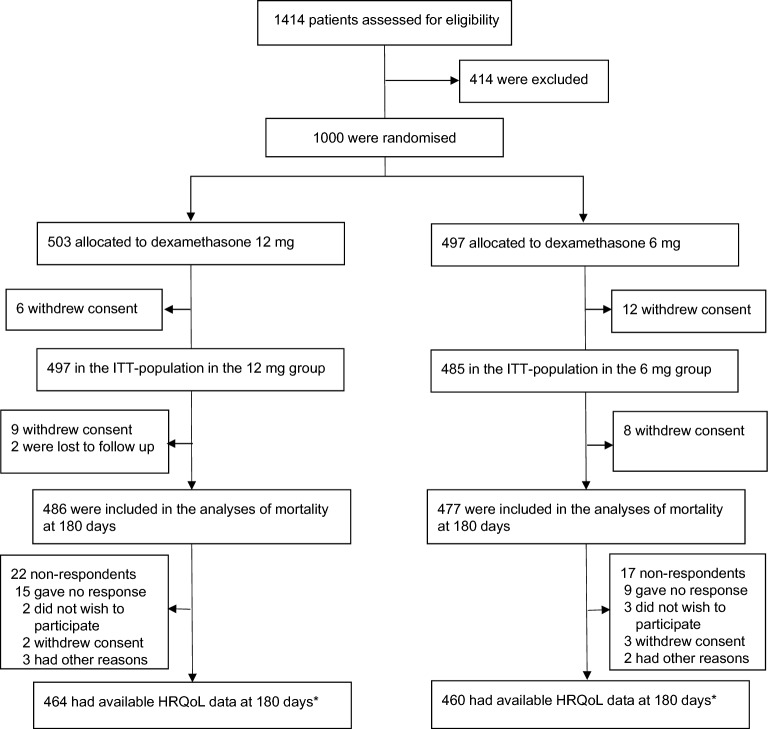
Fig. 1.
Patient flow in the COVID STEROID 2 trial. The details up to 90 days were presented in the primary report [4]. Eighteen patients withdrew consent for the use of any data (12 patients before the first dosing of trial medication and 6 after); the intention-to-treat (ITT) population therefore consisted of 982 patients in total. There were patient withdraws at three levels because of repeated follow-up of patients. *The primary HRQoL analyses were done in the ITT population (n = 982) with deceased patients assigned zero and missing data (n = 60 for EQ-5D-5L index values and n = 58 for EQ VAS scores) multiply imputed