Fig. 1.
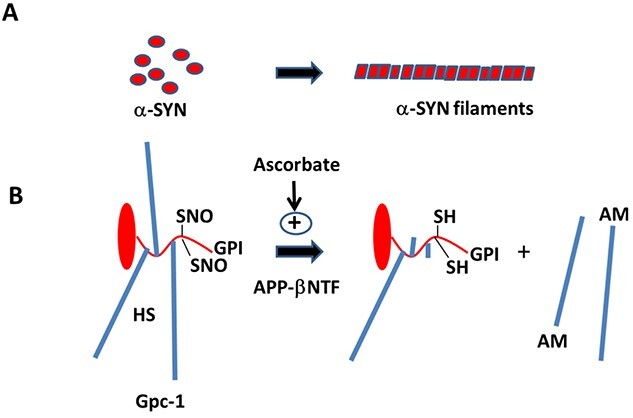
Aggregation of α-SYN to α-SYN filaments and NO-dependent deaminative release of HS from Gpc-1. (A) Conformational changes in the N-terminal domain of α-SYN monomers can induce filamentous aggregation. (B) The major part of the Gpc-1 protein (red) consists of an elongated N-terminal domain followed by a smaller flexible segment that is substituted with three HS chains (blue), contains two SNO cysteines and terminates with a GPI lipid anchor. The SNO groups participate in the deaminative release of HS, which is catalyzed by the N-terminal, β-secretase-released domain of the amyloid precursor protein (APP-βNTF) and is stimulated by ascorbate (vitamin C). The released HS chains contain reducing terminal anhydromannose (AM). SH, cysteine thiols.
