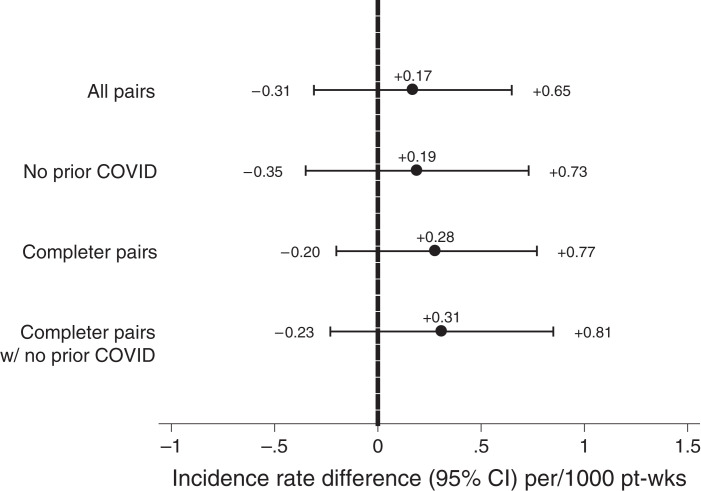
Figure 3.
IRDs for Ad26.COV2.S versus BNT162b2. Rates are presented for all matched pairs in the primary analytical cohort, those pairs with no history of COVID-19 (i.e., preceding index date), completer pairs (pairs for which the patient receiving BNT162b2 completed the two-dose series), and completer pairs with no history of COVID-19. Rates are presented per 1000 pt-weeks at risk. Confidence intervals crossing the null (0, vertical dashed line) indicate no statistically significant difference between Ad26.COV2.S and BNT162b2.