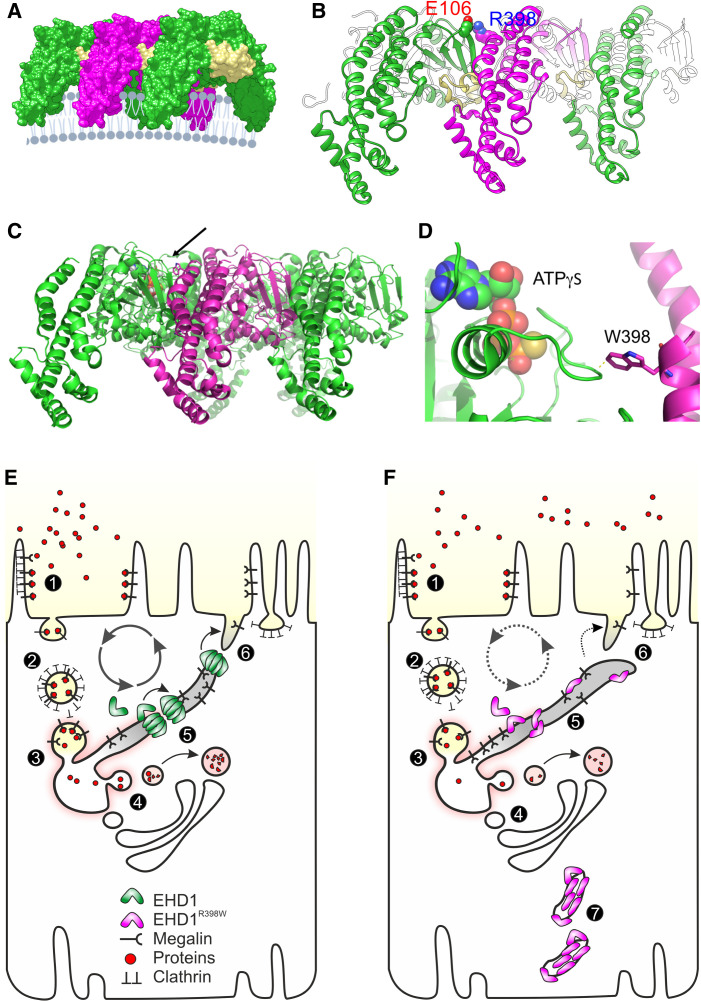
Figure 7.
Structural consequences and cell model. (A) Three copies of the activated EHD1 homology model were arranged as oligomer similar to the packing of EHD4 in the crystal (Protein Data Bank entry code 4CDI). A potential arrangement of the EHD1 oligomer (here trimer of dimers) at the membrane is visualized. The KPF loop (colored in yellow) of one dimer facilitates major dimer-dimer contacts allowing for a repetitive back-to-front arrangement of EHD1 dimers. (B) Homology modeling suggested that Arg398 located at the tip of α12 faces toward the dimer interface. A possible interaction that would contribute to the dimer interactions is Glu106-Arg398, which would further stabilize the interaction of the KPF loop (Pro110-Arg135) with α12. (C) Side-on view of oligomeric form of EHD4. Membrane binding is predicted to be at the bottom. The arrow indicates the position of the mutated residue at equivalent position 398 in EHD1. (D) Zoomed view of the tryptophan (W) at the equivalent position 398 in human EHD1. The W is shown in stick representation, projecting close to a loop of the nucleotide-binding pocket. ATPγS is shown bound in this pocket in space-filling representation. Note the close proximity of the W residue, which is likely to constrain nucleotide binding, hydrolysis, or release by EHD1. (E) Simplified model of endocytosis and recycling of membranes and receptors in normal proximal tubules. (1) At the base of the brush border membrane, after megalin and cubilin (not shown) bind their ligands, such as filtered proteins, the plasma membrane forms invaginations, mostly via Clathrin-coated pits, which lead to the formation of endosomes (2). The endosomes are further processed into sorting endosomes (3) and the endocytic recycling compartment. Low pH in vacuoles contributes to the separation of ligands from their receptors (4). From the endocytic vacuoles/recycling endosomes, these dissociated ligands are directed to multivesicular bodies (not shown) and finally to lysosomes. Receptor-containing vesicles are redirected to the apical membrane (via dense apical tubules). EHD1 and its dimers/oligomers are involved in fission of the endocytic tubules (5) and support receptor recycling (6). (F) Tubule fission is impaired in EHD1 patients and knockin mice. Mutant EHD1 dysfunction slows the recycling rate and leads to renal tubular proteinuria, as demonstrated in affected individuals and in animal models. In addition, mutant EHD1 is found in tubular structures (7) in the cytosol. Megalin and cubilin are not found in these structures, suggesting that EHD1 does not physically interact with these receptor proteins.