Figure 4.
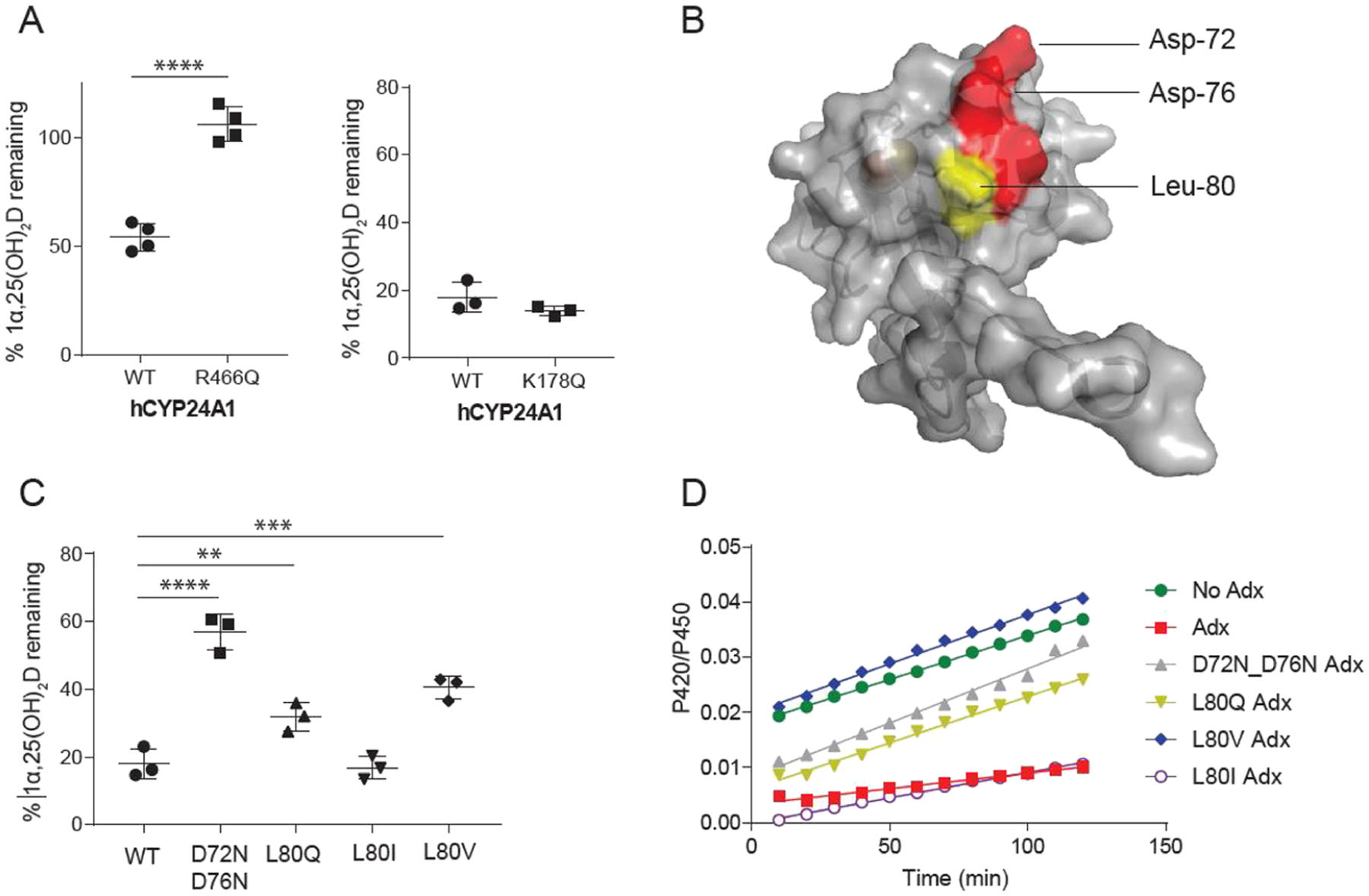
Effects of surface mutations of hCYP24A1 and hAdx. (A) 1α,25(OH)2D depleted in the presence of hCYP24A1 R466Q and K178Q. Reactions were run for 5 and 2.5 min each compared with a control of WT hCYP24A1. (B) Structure of hAdx (PDB 3P1M) with orientations of Asp-72 and Asp-76 indicated in red and Leu-80 in yellow. (C) 1α,25(OH)2D depleted in the presence of hAdx mutants. (D) Time-dependent change in the CO-bound spectra in the presence of each hAdx mutant. Asterisk (*), one-way ANOVA analysis α = 0.05, n = 3, p < 0.05.
