Table 4.
Post-Functionalization of C2-Alkenyl Sugar.a
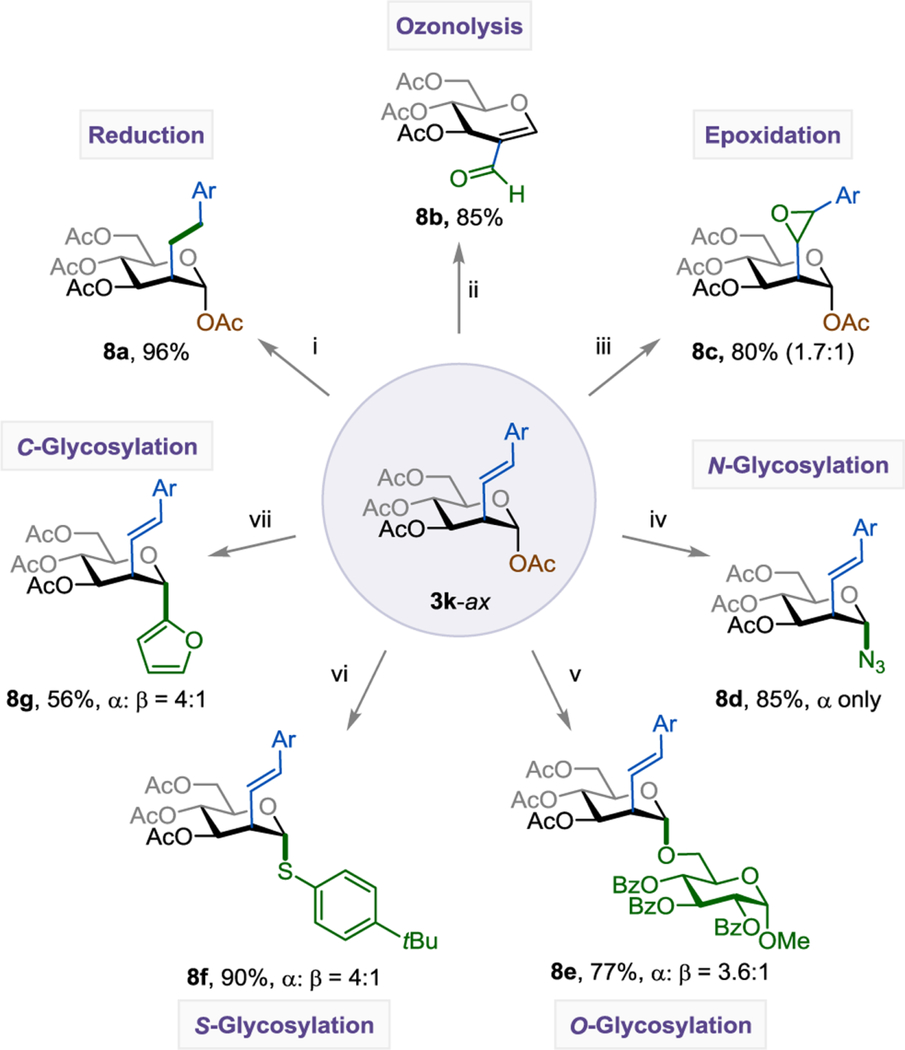
|
See SI for experimental details. The isolated yield and axial:equatorial (ax:eq) ratio are indicated below each entry. Reaction conditions: (i) Pd/C (10 mol%), EtOAc (0.100 M), H2 (1 atm), rt, 3h; (ii) 1. O3 (1 atm), DCM (0.100 M), –78 °C, 45 min; 2. PPh3 (2.00 equiv); (iii) m-CPBA (2.00 equiv), DCM (0.100 M), rt. 24h; (iv) BF3•Et2O (1.20 equiv), TMSN3 (1.20 equiv), DCM (0.100 M), 0 °C – rt, 2h; (v) BF3•Et2O (1.20 equiv), (2R,3R,4S,5R,6S)-2-(hydroxymethyl)-6-methoxytetrahydro-2H-pyran-3,4,5-triyl tribenzoate (1.20 equiv), DCM (0.100 M), 0 °C – rt, 2h; (vi) BF3•Et2O (1.20 equiv), 4-tert-butylbenzenethiol (1.20 equiv), DCM (0.100 M), 0 °C – rt, 2h; (vii) BF3•Et2O (1.20 equiv), furan (1.20 equiv), DCM (0.100 M), 0 °C – rt, 2h.
