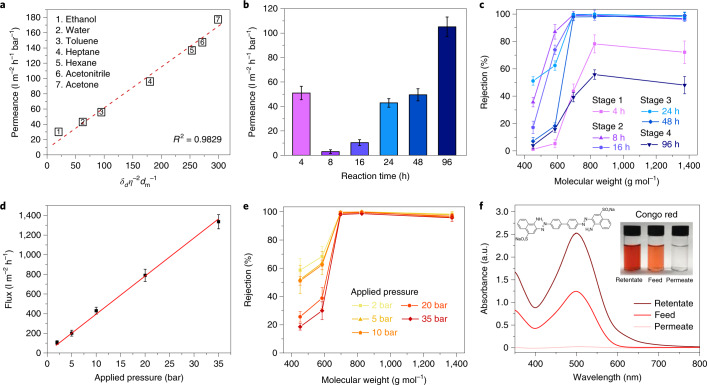
Fig. 3. Nanofiltration performance of CC3α membranes.
a, Plot showing pure solvent permeances versus their combined solvent properties (viscosity η, molar diameter dm and solubility parameter δd) for CC3α-PAN, where R2 is the coefficient of determination for the function. Hansen solubility parameter (δ) and the physical properties of each organic solvent are listed in Supplementary Table 2. b, Water permeance for CC3α-PAN-X h-0.8% membranes fabricated using reaction times that ranged between 4 and 96 hours. c, Dye rejection measurements for CC3α-PAN-X h-0.8% membranes in water. d,e, Water flux (d) and dye rejections (e) of a CC3α-PAN membrane under a range of applied pressures. f, Ultraviolet–visible absorption spectra of Congo red in water before (feed) and after (permeate and retentate) selectivity tests performed with CC3α-PAN. Insets show photographs of the feed, permeate and retentate solutions and the molecular structure of Congo red. Dye rejection was calculated using the intensity of the maximum absorption peak in the permeate and the feed, and equation (3) in the Methods. Mass balance calculations were performed using the maximum absorption peak values of the feed, permeate and retentate, with equation (4). All error bars depict the standard deviation (s.d.) of the data points obtained from at least three independent membranes.