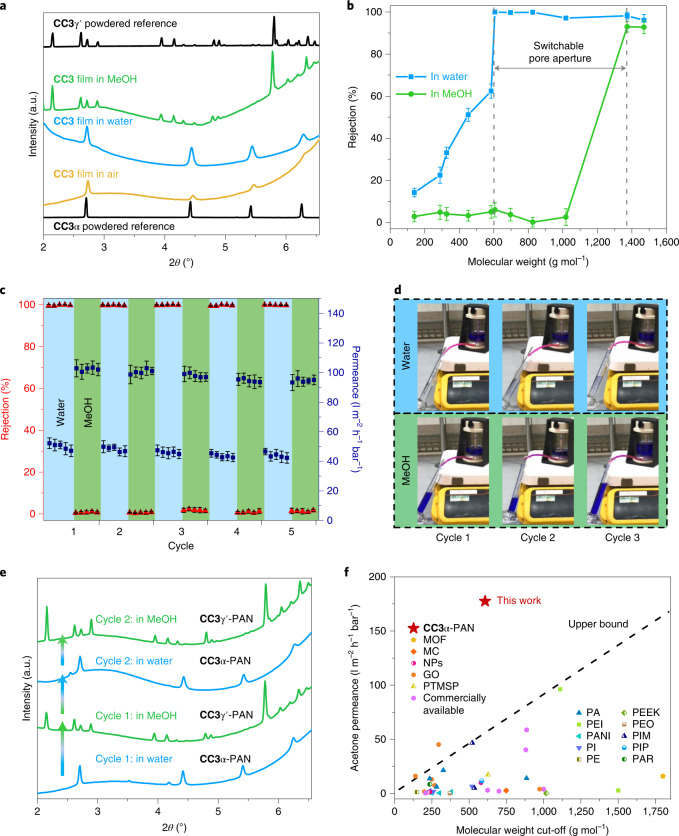
Fig. 4. X-ray diffraction characterization and switchable separation performance of CC3-PAN membranes.
a, GIXRD of CC3α-PAN in air and water, and CC3γ′-PAN in MeOH. Experimental PXRD patterns of CC3α and CC3γ′ powders are included as references. b, MWCO curve for CC3α-PAN in water and CC3γ′-PAN in MeOH containing 20 ppm dye solutes. The MWCO was determined by interpolating from the plot of rejection against the molecular weight of the dyes and corresponds to the molecular weight for which rejection reaches 90%. All error bars depict the s.d. of the data points obtained from at least three independent membranes. c, Reversible dye rejection of BB and solvent permeance of the CC3-PAN membrane observed upon switching the feedstock solvent between water and MeOH. All error bars denote the s.d. for measurements from at least three independent membranes. d, Photographs of CC3-PAN filtration dead-end cell captured from Supplementary Video 1 during the different cycles; BB is rejected in water by CC3α-PAN while CC3γ′-PAN does not reject BB in MeOH. e, In situ GIXRD patterns showing the reversible phase transition between CC3α-PAN and CC3γ′-PAN, by cycling between water and MeOH. f, Acetone permeance versus MWCO of general solutes in acetone for nanofiltration membranes reported in the literature and CC3α-PAN. MOF, metal–organic framework; MC, macrocycle; NPs, nanoparticles; GO, graphene oxide; PTMSP, poly(1-(trimethylsilyl)-1-propyne); PA, polyamide; PEI, polyethyleneimine; PANI, polyaniline; PI, polyimide; PE, polyethylene; PEEK, poly(ether ether ketone); PEO, poly(ethylene oxide); PIM, polymers of intrinsic microporosity; PIP, piperazine; PAR, polyacrylate (Supplementary Table 9 for full details).