Abstract
Some filoviruses can be transmitted to humans by zoonotic spillover events from their natural host and filovirus outbreaks have occured with increasing frequency in the last years. The filovirus Lloviu virus (LLOV), was identified in 2002 in Schreiber’s bats (Miniopterus schreibersii) in Spain and was subsequently detected in bats in Hungary. Here we isolate infectious LLOV from the blood of a live sampled Schreiber’s bat in Hungary. The isolate is subsequently sequenced and cultured in the Miniopterus sp. kidney cell line SuBK12-08. It is furthermore able to infect monkey and human cells, suggesting that LLOV might have spillover potential. A multi-year surveillance of LLOV in bats in Hungary detects LLOV RNA in both deceased and live animals as well as in coupled ectoparasites from the families Nycteribiidae and Ixodidae. This correlates with LLOV seropositivity in sampled Schreiber’s bats. Our data support the role of bats, specifically Miniopterus schreibersii as hosts for LLOV in Europe. We suggest that bat-associated parasites might play a role in the natural ecology of filoviruses in temperate climate regions compared to filoviruses in the tropics.
Subject terms: Viral reservoirs, Pathogens, Virus-host interactions
Lloviu virus (LLOV) is a filovirus that was first identified in 2002 in Schreiber’s bats in Europe. Here, the authors isolate infectious LLOV from Schreiber’s bats in Hungary and show that it can infect human cells in vitro, suggesting potential for zoonotic events. They furthermore detect LLOV RNA in ectoparasites of sampled bats.
Introduction
Some members of the Filoviridae family are known to cause severe disease and frequent death in humans (e.g., ebolaviruses and marburgviruses), while there are other members with no known pathogenicity to humans (e.g., Reston virus)1. Among filoviruses, ebolaviruses have received the most attention, both by public health experts and the public, mainly due to the multiple documented human outbreaks, particularly the West African Ebola virus (EBOV) disease outbreak in 2013–2016 and the recent outbreaks in the Democratic Republic of the Congo and Guinea2–4. The first direct evidence pointing towards bats as natural reservoirs for EBOV was published in 2005, when RNA and virus-specific antibodies were detected in three species of fruit bats captured in Gabon and the Democratic Republic of the Congo5. To date, direct isolation of the infectious virus from a specific bat species has only been achieved in the case of Marburg virus (MARV), which leaves several open questions regarding the natural reservoir host for other filoviruses6. During the past few years, increased scientific focus on these viruses has revealed the presence of several novel filoviruses in bats from Asia7–10, Africa11–13 and Europe14,15. Currently, the only filovirus known to be endemic in Europe is Lloviu virus (LLOV), where genomic RNA was identified during the investigation of Schreiber’s bats (Miniopterus schreibersii) die-off events in the Iberian-peninsula in 200214. After its initial discovery, no other reports were published with regards to the presence of this virus until 2016, when RT-PCR positive bat carcasses in Hungary confirmed a more widespread presence of LLOV in M. schreibersii across Europe15. This was followed by a report on seropositivity among Schreiber’s bats in Spain, suggesting the circulation of LLOV in that area16. The circumstances of the initial detection of LLOV in Hungary were similar to the original events in Spain, with multiple possibly related die-off events in the area, exclusively affecting Schreiber’s bats15. Nearly two decades have now passed since the discovery of LLOV and many questions remain unresolved regarding the nature of the virus, the risk of zoonotic spillover, and most importantly, the pathogenic potential for bats and humans. Concerns regarding the possible pathogenicity of LLOV in humans remain high, since multiple studies have revealed its functional and genomic relatedness to EBOV17–20. Recently, a recombinant LLOV (rLLOV) rescue system was developed, in which the missing LLOV genome termini were complemented by homologous EBOV or MARV sequences. Although rLLOV is able to infect known target cells of EBOV, including human primary macrophages, the inflammatory response in human macrophages, a hallmark of Ebola virus disease, is not induced by rLLOV. This suggests that LLOV might be able to infect humans, but the infection might not lead to disease, similar to the nonpathogenic Reston virus21. Public health preparedness to filovirus zoonoses is highly related to the ecological attributes of wildlife hosts. Outbreaks in the human population are most likely initiated by spillover events from infected animals to humans22. In the case of MARV, these spillover events have been reported to correlate with the life cycle of the bat host, with increased zoonotic events occurring during the birthing period23,24. Importantly however, bat life cycles are different in temperate climate conditions than in the tropics. The annual life cycle of Schreiber’s bats has key differences from bats that live in the tropics, most importantly the period of hibernation (Supplementary Fig. 1).
In the past two decades, the identification of reservoir species of filoviruses has become a major focus of research, with numerous different vertebrate and arthropod taxa being studied25–27. Interestingly, ectoparasites of bats have not been involved in many of these investigations, although they can carry several types of pathogens28. Bat flies are the most common ectoparasites of bats29. In the case of hippoboscoid flies, both sexes feed on the blood of the host species. Members of the Nycteribiidae and Streblidae families are typically associated with specific host species and in general bat flies and bats show a strong coevolution30. While bats are a major focus in viral emerging infectious disease research, the transmission patterns of these viruses within host bat populations and the role of their highly-specialized ectoparasites in this enzootic ecology have barely been investigated to date28. Ectoparasites, however, represent perfect vector candidates for bat-to-bat transmission, including intraspecies and interspecies transmission31. It is therefore important to understand the complex picture of LLOV transmission within bats and potential vector species, including ectoparasites, to get a clearer picture about the natural circulation of this, and other filoviruses.
In the present study, we sought to understand the biology of LLOV at a specific bat roost site in northeastern Hungary. We investigated the role of Schreiber’s bats in the natural circulation of LLOV and whether bat-associated parasites might play a role in the ecology of LLOV. We performed serologic, RT-PCR and sequencing-based surveillance on Schreiber’s bats from a site of previous LLOV detection in Hungary15 following a step-by-step investigation strategy. A primary result was the isolation of infectious virus directly from a bat blood sample, which makes LLOV only the third member of the family Filoviridae ever isolated from bats and the first filovirus isolation of a non-Marburg or Ebola virus genus member.
We developed and used a deployable field-sequencing technology based on target enrichment method. It is not without precedent, as similar techniques were used extensively to sequence viruses from patient samples during the West African EBOV outbreak32,33. Here, we publish the use of such technology directly on wildlife hosts, optimized for the Nanopore sequencing platform that is amenable for both field and laboratory use in future studies.
Results
LLOV seropositivity in Schreiber’s bats
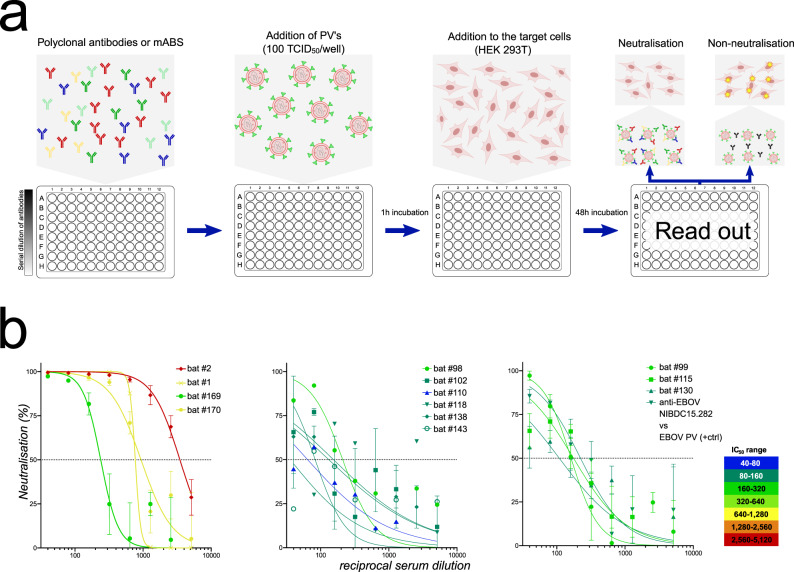
Blood samples were collected from live bats and bat carcasses during the period of 2016–2020 at the site of LLOV detection in Northeastern Hungary in 201615. Live bats were apparently healthy, whilst dead bats were found in various conditions, possibly depending on the incubation period of the carcass within cave conditions. All details about the samples and animals, such as sex and collection date are listed as Supplementary Data 1. In order to perform neutralization tests to detect the presence of anti-LLOV antibodies, we created lentivirus particles pseudotyped with the LLOV or EBOV glycoprotein (GP). LLOV PVs did not cross react with EBOV convalescent serum (NIBSC WHO standard 15/262) (Supplementary Fig. 4). The converse neutralizing cross-reactivity was tested using bat sera and an EBOV PV target. However, due to the insufficient remaining volumes of bat sera only two LLOV nAb positive bat sera and two LLOV nAb negative bat sera could be tested, in addition to EBOV convalescent serum as a positive control. Importantly, no cross-reactivity was observed in these experiments (Supplementary Fig. 4). These findings are in accord with the lack of inter-genera filovirus cross-reactivity previously reported between Ebola and Marburg viruses using an indirect ELISA34.
Following the cross-reactivity tests with EBOV, we used the LLOV PVs to perform antibody neutralization tests (PVNTs) on bat serum samples from both live and dead bats (Fig. 1, Table 1). Nine Schreiber’s bat carcasses were collected during the period between 2016 and 2019 and from these four tested seropositive for LLOV, with relatively high neutralization titres (254–3485 IC50) (Supplementary Data 1). We were also able to detect LLOV RNA in certain tissues of these carcasses with low copy numbers, detailed in Tables 1, 2. We also investigated serum samples taken from 74 live Schreiber’s bats from the first sampling event in September 2018. Seroprevalence among live-sampled bats was 9/74 (12.16%) with relatively weak to moderate titres (64–211 IC50), particularly compared to seropositive carcases (Fig. 1, Table 1). Unfortunately, the highly limited amount of blood samples from live animals precluded replicate testing in certain cases, also hindering the possibility of coupled serologic and RT-PCR examination. Therefore we were able to conduct both serological and RT-PCR examination in only one case (LLOV_105) where LLOV RNA was detected along with seronegativity (Supplementary Data 1).
Fig. 1. Pseudotyped virus neutralization test (PVNT) experiments of blood sera from Schreiber’s bats.
a Workflow of PVNT; b PVNT results of LLOV seropositive bat sera. First graph represents dead animal samples (samples: bat #2, bat #1, bat #169, bat #170), whilst the second and third graph presents live bat samples (bat #98 to bat #130). Anti-EBOV NIBSC15.282 vs. EBOV PV data is also shown as a matched positive technical control. Error bars indicate mean ± SD. Source data are provided as a Source Data file.
Table 1.
PVNT results of seropositive bats paired with RT-PCR results.
| Animal ID | PVNT LLOV 1 IC50 | PVNT LLOV 2 IC50 | Mean IC50 | Serology | Real-time RT-PCR | Status | Collection date |
|---|---|---|---|---|---|---|---|
| LLOV 98 | 211 | a | 211 | Pos | N/A | Alive | 2018.09.18. |
| LLOV 99 | 156 | 174 | 165 | Pos | N/A | Alive | 2018.09.18. |
| LLOV 102 | 132 | a | 132 | Pos | Neg | Alive | 2018.09.18. |
| LLOV 110 | 64 | a | 64b | Pos | Neg | Alive | 2018.09.18. |
| LLOV 115 | 143 | 197 | 170 | Pos | N/A | Alive | 2018.09.18. |
| LLOV 118 | 155 | a | 155 | Pos | Neg | Alive | 2018.09.18. |
| LLOV 130 | 177 | 59 | 118 | Pos | Neg | Alive | 2018.09.18. |
| LLOV 138 | 83 | a | 83 | Pos | Neg | Alive | 2018.09.18. |
| LLOV 143 | 90 | a | 90 | Pos | Neg | Alive | 2018.09.18. |
| LLOV 1c | 768 | N/A | 768 | Pos | Pos (lung, spleen) | Dead | 2016.02.11. |
| LLOV 2c | 2999 | 3972 | 3485 | Pos | Neg | Dead | 2016.02.11. |
| LLOV 169c | 172 | 337 | 254 | Pos | Pos (lung, spleen) | Dead | 2019.01.31. |
| LLOV 170c | 757 | 1136 | 946 | Pos | Neg | Dead | 2019.01.31. |
| Control 1 | Non-neut. | Non-neut. | Neg | Neg | Alive | 2019.08.08. | |
| Control 2 | Non-neut. | Non-neut. | Neg | Neg | Alive | 2019.08.08. |
aPVNT not performed due to lack of sufficient serum for a repeat experiment.
bBorderline positive value.
cDead at the original site of virus emergence, Northeast Hungary, Zemplén Mt.; Control 1 (Myotis myotis) and Control 2 (M. schreibersii) were used as healthy negative control animals from a distant bat roost site in Southwestern Hungary.
Table 2.
Detailed information about LLOV RNA-positive samples.
| Sample ID | Species | Collection date | Tissue(s) | Cycle threshold (Ct) | Genomic copy numbers (copies/mL) |
|---|---|---|---|---|---|
| LLOV_1a | Miniopterus schreibersii | 11.02.2016 | Lung; spleen | 35.56; 35.30 | 1.4 × 106; 1.6 × 106 |
| LLOV_5a | Miniopterus schreibersii | 11.02.2016 | Lung | 35.09 | 1.8 × 106 |
| LLOV_105 | Miniopterus schreibersii | 18.09.2018 | Blood | 26.16 | 2.3 × 108 |
| LLOV_105_P2 | Nycteribia schmidlii (P) | 18.09.2018 | Whole specimen | 34.08 | 3.2 × 106 |
| LLOV_147 | Miniopterus schreibersii | 19.09.2018 | Blood | 25.39 | 3.6 × 108 |
| LLOV_147_P2 | Nycteribia schmidlii (P) | 19.09.2018 | Whole specimen | 34.61 | 2.4 × 106 |
| LLOV_169a | Miniopterus schreibersii | 31.01.2019 | Lung; spleen | 38.59; 36.31 | 2.7 × 105; 9.4 × 105 |
| LLOV_329b | Miniopterus schreibersii | 23.09.2019 | Blood | 30.27 | 2.5 × 107 |
| LLOV_329_P1 | Ixodes simplex (P) | 23.09.2019 | Whole specimen | 36.10 | 1.1 × 106 |
| LLOV_329_P2 | Nycteribia schmidlii (P) | 23.09.2019 | Whole specimen | 35.78 | 1.3 × 106 |
| LLOV_378b, c | Miniopterus schreibersii | 24.09.2019 | Blood | 30.10 | 2.8 × 107 |
| LLOV_378_P1c | Nycteribia schmidlii (P) | 24.09.2019 | Whole specimen | 34.53 | 2.5 × 106 |
(P) ectoparasite.
aCarcasses.
bSample was used for in vitro isolation efforts.
cSamples were subjected to viral genomic sequencing.
LLOV RNA in bats and related ectoparasites
After the verification of seropositivity in bats, we focused on detection of LLOV RNA via RT-PCR in consecutive sampling events to facilitate viral genome sequencing and virus isolation efforts. Regular checking for dead animals during the hibernation period resulted in 10 bat carcasses (nine Miniopterus schreibersii, one Myotis myotis). After dissection of these carcasses, we tested blood and multiple organ samples (brain, liver, lung, spleen, and kidney) for the presence of LLOV and detected viral RNA in two (2/5) dead bats from 2016 and one from 2018 (Table 2). Overall, LLOV prevalence among Miniopterus carcasses was 33.3% (3/9). Notably, LLOV RNA was only detected in spleen and lung samples, with low genomic copy numbers in both organs comparing to live bat samples where we found one to three orders of magnitude higher amounts of viral genomic copies (Table 2).
Altogether, 779 samples (351 blood, 89 feces, 19 urine, 320 ectoparasites) were collected from live animals (351—Miniopterus schreibersii, 2—Myotis myotis) in seven sampling events and tested for the presence of LLOV RNA (Fig. 2a). We performed on-site RT-PCR analysis for the detection of viral RNA in blood samples from live animals, while other sample types were tested under laboratory conditions. On-site RT-PCR permitted the targeted examination and re-sampling of RT-PCR positive bats. Overall, 1.14% (4/351) of live-sampled animals were positive for LLOV by RT-PCR. Only the mid-to-late September sampling of bats in 2018 (LLOV_105, LLOV_147) and 2019 (LLOV_329, LLOV_378) provided positive blood samples, with a 4% RT-PCR positivity rate in 2018 and 2.8% in 2019 (Fig. 2). We did not detect LLOV RNA-positive animals in 2020, but only limited sampling was conducted due to COVID-19 pandemic-related restrictions. None of the RT-PCR positive animals showed signs of disease, and their general condition and activities appeared normal.
Fig. 2. Summary of tested bat samples, verified RT-PCR positive bats and their ectoparasites.
a Number of LLOV-specific RT-PCR negative (lighter color) and positive (darker color) samples in each category: blood or organs (blue), feces (brown), urine (yellow), ectoparasites (green). *During these sampling events only passive monitoring was performed; (b) schematic representation of coupled bat-ectoparasite LLOV RT-PCR positivity.
We collected feces samples from an RT-PCR positive bat (LLOV_378) to test the shedding characteristics of the virus and found no evidence of the presence of LLOV RNA in these samples (Fig. 2). All other feces (n = 89) and urine (n = 19) samples were retrieved from animals with RT-PCR negative blood and were found to be negative as well.
In order to get a clearer picture of possible bat-to-bat transmission pathways, we also examined the presence of LLOV RNA in bat-related ectoparasites, mostly from the family Nycteribiidae known as “bat flies” along with some hard ticks (Ixodidae). In the process of collecting bat samples, specimens of bat flies including Nycteribia schmidlii (n = 214), Nycteribia latreillii (n = 2), Penicillidia conspicua (n = 65), and Penicillidia dufouri (n = 4), as well as Ixodes simplex (n = 33) and Ixodes vespertilionis (n = 2) ticks were collected. Positive ectoparasites were only retrieved from positive bats, suggesting the presence of the virus exclusively in engorged parasites (Fig. 2, Supplementary Data 1). Altogether, we detected LLOV RNA in four Nycteribia schmidlii samples and one Ixodes sample from four RT-PCR positive bats (Fig. 2b).
A detailed summary of all RT-PCR positive samples, including sample type/organ, CT values of RT-PCR reactions and indications of follow-up experiments are presented in Table 2.
Lloviu virus isolation on bat, monkey, and human cells
Freshly obtained blood samples from the animals previously denoted PCR positive (LLOV_329, LLOV_378) were used for in vitro isolation experiments. Considering the small body size of these bats, the volume of these samples is routinely extremely small, usually between 5 and 20 µl. The initial in vitro isolation efforts, utilizing multiple cell lines (Vero E6—African green monkey kidney and Tb1-Lu—bat lung cells) did not result in detectable LLOV replication. We neither observed any cytopathic effect in the cell culture experiments nor obtained positive real-time RT-PCR results after three blind passages.
After obtaining SuBK12-08 cells35, we repeated these efforts using the only remaining LLOV_378 blood sample, since by this time we had used most of the positive sample aliquots in previous isolation efforts. SuBK12-08 is a Miniopterus schreibersii-originated, SV40 transformed kidney cell line. After the second blind passage, we observed a strong cytopathic effect (Fig. 3). Virus replication was further verified by real-time RT-PCR which resulted in lower real-time Ct value (18.469) than the original blood sample (30.1), clearly indicating an increase in viral genomic copy numbers. Viral titers of passage 4 virus were determined by tissue culture infectious dose (TCID50) assay on SuBK12-08 cells and were as high as 1.78 × 109 TCID50 units/ml. The established virus isolate was subjected for in vitro testing of other cell lines. Vero E6, SH-SY5Y (human neuroblastoma), Hep G2 (human hepatocyte carcinoma), HCC-78 (human lung adenocarcinoma), HCT 116 (human colon carcinoma) were successfully infected with MOI 0.01 virus isolate. After 10 days post-infection we measured a significant increase in viral genomic copy numbers. RT-PCR results of the cell supernatants are given (Supplementary Table 1). We present the visual progression of CPE on the SuBK12-08 (Supplementary Movie 1) and Vero E6 (Supplementary Movie 2) cell lines during LLOV infection.
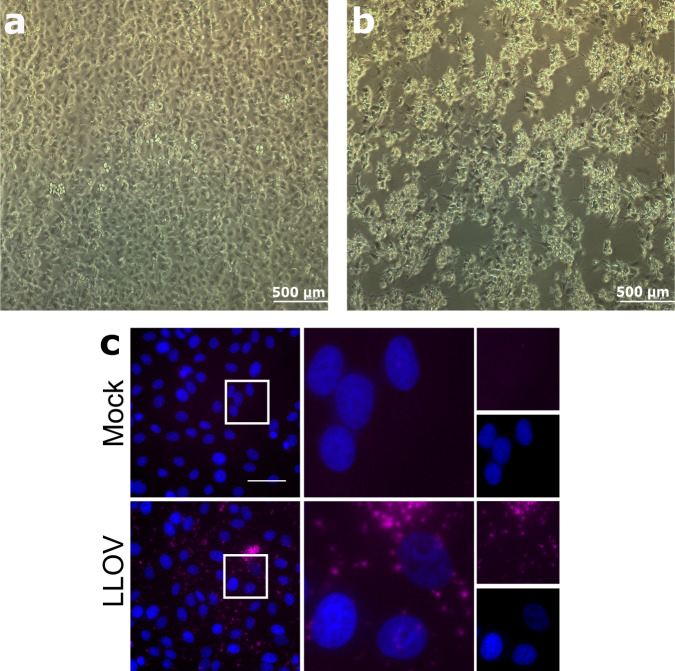
Fig. 3. In vitro characterization of the Lloviu virus isolate.
Blind passage of Lloviu virus isolate and cytopathic effect on SuBK12-08 cells 10 days post infection (DPI), passage 4 (a, b) In situ hybridization of the Lloviu virus isolate on Vero E6 cells (c). a mock-infected cells 10 DPI, (b) Lloviu virus-infected cells 10 DPI, (c) Vero E6 cells were left uninfected (mock) or infected with LLOV at an MOI of 3. At 1 day post-infection, cells were fixed and stained by RNA FISH for viral genomic RNA (magenta). Cell nuclei were stained with DAPI (blue). Top row, mock-infected; bottom row, LLOV- infected. Inset square in left panels indicates region magnified in middle panels; far right panels display individual channels from middle panels. Scale bar represents 50 µm.
Using the Vero E6 cell line, RNA fluorescent in situ hybridization (FISH) staining with LLOV genome-specific probes revealed a punctate pattern in LLOV-infected cells that was not observed in mock-infected control cells, confirming the presence of replicating LLOV in the infected cells (Fig. 3).
Sequencing of the viral genome
We developed an ARTIC-like amplicon-based (https://artic.network/) method for sequencing the complete coding region of the LLOV genome32,36,37. Using this method, we were able to retrieve sequence data from the RT-PCR positive bat from which virus was isolated (LLOV_378) and a nearly complete genome from its associated bat fly ectoparasite (LLOV_378_P1) (Table 2).
We generated sequence data for ~99% of the known LLOV reference genome (NCBI accession NC_016144). In the case of sample LLOV_378, 99.45% of the known genome was covered, starting from reference nucleotide position 28 and ending at 18,853. The mean coverage of this sample using all the data which was derived from the three different primer sets was 17,345× (Supplementary Fig. 2). The consensus sequence derived from the RT-PCR positive-associated parasite, sample LLOV_378_P1, spanned positions 28 to 18,853, with one notable gap between position 11,937 and 12,051. Importantly, there were no differences between the bat and bat fly derived viral sequences. Our LLOV sequence data are 99.198% identical to the reference genome. We detected the highest level of sequence variation between the sequence data and the reference genome within the glycoprotein gene 2 (GP2) (Fig. 4). We also performed an indicative sequencing of the established infectious isolate. The obtained genomic information for this isolate is available under the accession number (NCBI accession: MZ541881).
Fig. 4. Genomic and phylogenetic attributes of the Lloviu virus genome sequences presented in this manuscript.
a Annotated Lloviu virus genome sequence from bat derived sample LLOV_378 (Genbank MW775011). Blue bars indicate differences in amino acid sequences and the green bar represents an insertion compared to the Spanish LLOV reference isolate (GenBank: NC_016144); (b) summary table of amino acid coding differences between LLOV_378 and NC_016144; (c) phylogenetic analysis of select complete genome sequences representing four filovirus genera including LLOV_378 (Genbank: MW775011) and LLOV_378P1 (Genbank: MW775010). Sequences are colored by genus Dianlovirus (blue), Marburgvirus (green), Cuevavirus (orange), and Ebola virus (yellow)). Host Taxon name abbreviations: Ro. Rousettus, Ho. Homo, Mi. Miniopterus, Ma. Macaca, Mo. Mops, Ch. Chaerephon.
Discussion
Several novel members of the family Filoviridae have been discovered in the last decade including some found in Europe and Asia, raising the potential for the emergence of filoviruses outside of Africa. There is a considerable knowledge gap regarding the pathogenicity, animal hosts, and transmissibility of these newly discovered viruses.
The most groundbreaking result presented here is the successful isolation of infectious LLOV, from the blood sample of an RT-PCR positive bat. The pronounced cytopathic effect of LLOV-infected Miniopterus SuBK12-08 cells combined with the high titers of virus stocks propagated in these cells suggest that SuBK12-08 cells are highly permissive to LLOV infection. This, along with our other results, provides the first indication for a possible reservoir role of Schreiber’s bats, which warrants further investigation to establish whether these bats can serve as asymptomatic hosts for the LLOV in Europe. The isolation of the virus opens the possibility for extended pathogenicity studies in the near future. As we also present here, Lloviu virus has the potential to infect human cells, therefore receptor identification, gene expression and indeed antiviral studies are now a priority.
We describe the circulation of LLOV in a colony of Schreiber’s bats over the span of several years. This study represents the first detailed observation of the natural circulation of a filovirus in a temperate climate region. In addition to virus isolation results, the seropositivity and presence of LLOV RNA in these bats and the continuous circulation of the virus are supportive for the putative role of Schreiber’ s bats as the natural reservoir hosts for this virus38. However at this point we can only conclude these animals as hosts for the virus in Europe. Our observations are partially in line with the previous finding that Egyptian fruit bats (R. aegyptiacus) are a reservoir for marburgviruses39,40. Other bat species have been reported as hosts for other filoviruses, such as the Bombali virus11. We detected LLOV RNA in multiple deceased bats, raising the possibility that these bats died as a consequence of infection. Interestingly, some individuals were found with respiratory tract bleeding, however there was no correlation to RT-PCR positivity (Supplementary Table). Notably, the viral load in dead animals were one to three order of magnitude lower than in the live animal samples. More sophisticated surveillance activities are necessary to better understand the nature of LLOV infection in bats and to clarify the possible role of the virus in the morbidity and mortality among these bats. Although rare, the occurrence of both fatal and non-fatal outcomes of viral infections of bats is not unique. The best known pathogens to cause such patterns in bats are lyssaviruses41.
We also developed a sensitive serological test based on pseudotype virus neutralization that supports LLOV serosurveillance testing of bat sera samples. Before this study, only immunoblot analysis had been used for LLOV serologic examination in bats16. Our method enabled the serological study of live-sampled wild bats, showing a LLOV seroprevalence of 12.2% (9/74), which is lower than the previously published 36.5% seroprevalence from bats in Spain, though our test measures antibody neutralization rather than simply binding activity. This is also direct evidence of LLOV exposure in these bats, which supports the previous observation of a more extended spatio-temporal presence of the virus in Europe15.
To exclude possible cross-reactivity with different filovirus nAbs we performed cross-reactivity tests (Supplementary Fig. 4). Based on the numerous European studies, involving viral metagenomics or targeted surveillance there were no any indication for the presence of other filoviruses than LLOV in Europe so far42. In addition to these, during this study we performed a secondary, pan-filovirus nested RT-PCR screening on our samples (Supplementary Data 1)7. We found no evidence for the circulation of other filoviruses than LLOV within the colony. Based on these data we do not expect the presence of other filoviruses which may interfere with the neutralization experiments. Also, we used GP-based PVNA system which was previously described as the most specific antigen compared to NP and VP40 antigens of filoviruses43,44.
Except for MARV in Egyptian fruit bats, there is little information about enzootic circulation of a filovirus among specific bat colony sites. MARV virus prevalence values seen in various countries range from 2.5% and 13.7%, with seasonal variability noted6,23,45. Based on previous studies, natural fluctuation of the age composition within a single colony and therefore the changing numbers of susceptible animals within the population may largely affect the prevalence23. In case of Pteropodidae (fruit bats), bi-annual birth pulses may facilitate the persistence of filoviruses24. Available data on MARV circulation highlighted juvenile bats (around ~6 months old) as particularly likely to be infected at specific times of the year. This seasonal emergence of juvenile Egyptian fruit bats coincides with increased risk of human infection23. In contrast to some tropical species, M. schreibersii bats have only one breeding season per year, and notably have a hibernation period. Considering these features, there should be other driving factors involved in LLOV circulation (Supplementary Fig. 1).
The hibernation may also alter the response to LLOV infection as reactivation of latent herpesvirus infection or prolonged coronavirus infection were both reported in bats in previous studies46. At this point it is hard to assess the causality of LLOV infection and fatal hibernation outcome in these bats, it is thus crucial to understand possible hibernation effects of LLOV infection to better understand the pathogenic potential of this virus to these bats.
We have also provided evidence of filovirus RNA presence in arthropods. However, the precise role of bat flies and other ectoparasites in LLOV transmission is unknown. It is not yet clear whether these bat flies may be acting as natural vectors or mechanical vectors for LLOV transmission or are simply dead-end LLOV spillover hosts. A recent article reported the unsuccessful infection of bat flies with MARV, ruling out the biological vector competence but still considered the possibility of mechanical transmission47. Our observations are in accordance with this recent report, since LLOV-positive ectoparasites were only retrieved from positive animals, suggesting the presence of the viral RNA in engorged parasites. Coupled sequencing results from host and parasite also support passive transmission without active viral replication, since no differences were found in the viral genomic sequences derived from the bat fly (LLOV_378_P1) and its bat host (LLOV_378). Given the current relatively limited dataset, it is not clear whether these ectoparasites play a role as a vector in the transmission of LLOV. However, we cannot rule out the possibility that these parasites serve as mechanical vectors in the transmission of LLOV, which warrants further studies. Compared to previous studies, Reston virus was not found to replicate in arthropod vectors and MARV RNA was not found in parasites on MARV-positive bats48–50. Based on the successful sequencing of LLOV RNA isolated from bat flies, resulting in high-quality sequence data, indirect LLOV surveillance could potentially be conducted by testing ectoparasites of M. schreibersii bats, obviating the need for invasive blood sampling of bats. This would provide the opportunity to conduct wide scale geographic surveillance to discover LLOV endemic regions and obtain a more detailed distribution map of LLOV infection among M. schreibersii populations. The reliability of ectoparasite-based surveillance strategy of bats was already reported for multiple bacterial species51.
Based on our sequence data, we have shown insights into the natural genomic evolution of LLOV. Comparing the LLOV genomic sequence data from this study with the reference genome sequence from Spain, the level of LLOV sequence variation is consistent with other filovirus genera. For ebolaviruses and marburgviruses, the GP gene is a hotspot of nucleotide sequence variation52,53. The consistency of elevated GP sequence variation compared to other genomic regions in all three genera (Ebola virus, Marburgvirus, and Cuevavirus) suggests similar genetic evolutionary driving forces on this region (e.g., immune evasion). Based on the presented data and considering the previous reports from Spain14,16, there are multiple reports of LLOV infection in Schreiber’s bats, consistent with the idea that this species may serve as a natural reservoir of the virus, however at this point we can only conclude the role of these animals as hosts for the virus in Europe. Despite many remaining questions, we provide evidence and further support for the role of bats in the natural circulation of filoviruses in general. The transmission cycle of LLOV may be complex and potentially different from filoviruses whose enzootic cycle occurs in tropical climates. As a major difference, we present data showing LLOV RNA-positive highly-specialized bat-associated ectoparasites, highlighting the importance of future research regarding the role of these insects in LLOV and other filovirus transmissions. Similar surveillance studies may greatly facilitate the discovery and understanding of novel filoviruses. Most importantly, we have isolated the infectious virus which represents the only filovirus that does not belong to the ebola- or marburg-virus genera, and it is the third successful isolation of a filovirus directly from bats, following MARV and RAVV virus6, opening the door to further virological studies. Importantly, we show that multiple human cell lines are permissive to LLOV infection, which raises concerns about potential bat to human spillover events with LLOV in Europe and urges immediate pathogenicity and antiviral studies.
Methods
Ethics and biosafety statement
Bat sampling activities on a country-wide scale were approved by the Hungarian Government Office of Pest County under the registration number of PE-KTFO/4384-24/2018. Animal handling was performed by licensed chiropterologists, no animals were harmed during the study, and all ethical standards were followed during the work. All in vitro virus isolation procedures were performed under Biosafety Level 4 conditions within the laboratory of Szentágothai Research Center, University of Pécs, Hungary.
Animal sampling
We conducted regular bat colony monitoring during the hibernation period (between November and March) (passive surveillance) at the site of LLOV re-emergence in Hungary (Northeast Hungary, Zemplén Mountains) from 2016 to 2020, collecting only deceased bats, if found. Whole carcasses were frozen and transferred in a dry shipper to the biosafety level 4 (BSL-4) laboratory at the Szentágothai Research Center, Pécs, Hungary, where they were dissected. Lung, spleen, kidney, liver, and brain tissues as well as blood samples were stored at −80 °C until further analyses.
In addition, seven live animal sampling events (active surveillance) were performed between 2018 and 2020, when samples (blood, urine, feces, ectoparasites) were collected from Schreiber’s bats (Miniopterus schreibersii) and Greater mouse-eared bats (Myotis myotis). Bat species identification was performed by trained chiropterologists according to morphological identification keys54. Considering conservational aspects, all sampling activities were conducted after the reproduction period of the colony (between August to November). During the live sampling events, serum samples were taken from captured bats after which they were left hanging separately in disposable paper bags (air permeable) for ~2 h, while on-site RT-PCR analysis for the presence of LLOV RNA in from blood samples was performed (as detailed in the Virus detection section). This methodology permitted the observation and re-sampling of infected bats. Altogether, 376 bat individuals were sampled between 2016 and 2020 (Supplementary Table). Whole blood (maximum of 50 µL) was taken from the uropatagium vein from each animal by Minivette® POCT (Sarstedt, Germany) disposable microtubes. At the first live animal sampling event (18.09.2018), the blood samples were collected in 1.5-ml Eppendorf tubes, where serum was separated for neutralization assays by low-speed centrifugation (1000 × g) for 5 min. Cell pellets were used for nucleic acid extraction and RT-PCR detection of LLOV RNA. Due to the multipurpose nature of the investigation and the strong limitation of the blood amount, in case of samples less than 8 μL, only LLOV RNA detection was conducted. When more blood was collected (~8–13 μL), only serology was performed. For samples with volumes above 13 μL, both LLOV RT-PCR and serology testing were performed.
Following the first live sampling, we changed the blood sampling methodology in order to provide the possibility of in vitro isolation efforts. During the following six live sampling events (02.08.2019–18.09.2020), freshly obtained blood samples were transferred directly to 200 μL Virus Transport Medium (VTM) (UniTranz-RT 1 mL Transport System, Puritan, USA), and mixed gently by pipetting. A 100 μL portion of each sample was deposited for nucleic acid extraction while the remainder was stored and served as inoculum for virus isolation experiments. When possible, urine, feces, and ectoparasites (ticks, bat flies) were also collected with forceps during bat handling. Feces and urine samples and ectoparasite specimens were immediately individually frozen in liquid nitrogen, and stored at −80 °C until further laboratory processes. Ectoparasites of the Nycteribiidae family were identified based on morphological characteristics, identifying parasite species or genus according to generally approved reference keys using a stereomicroscope55,56. Tick samples were barcoded by sequencing the mitochondrial 16S rDNA sequence57.
Virus detection
For LLOV RNA detection, we performed RT-PCR tests from all sample types (lung, spleen, kidney, liver, brain tissues and blood from carcasses; blood, urine, feces samples and ectoparasite specimens from live bats). For blood samples in VTM, we carried out on-site LLOV RNA detection in the field, while other samples were transported to the laboratory for testing. These blood samples were further screened with a pan-filovirus nested-RT-PCR, previously used to discover several novel filoviral sequences in China58.
Tissues, feces, and ectoparasites were homogenized in 100 μL 1x Phosphate Buffered Saline (PBS) using Minilys Personal Homogenizer (Bertin, Germany) with glass beads. The urine samples were complemented with 1× PBS to a total volume of 100 μL. Then, the samples were centrifuged at 16,000 × g for 5 min and 100 μL supernatant was transferred for RNA extraction. The 100 μL blood samples in VTM were directly used for RNA extraction.
RNA was extracted using Direct-Zol RNA MiniPrep (Zymo Research, USA). RNA was then used for RT-PCR using the QIAGEN OneStep RT-PCR kit (Qiagen, Germany) at 50 °C for 30 min, and 95 °C for 15 min, followed by 45 cycles of 95 °C for 15 s, 60 °C for 20 s 72 °C for 40 s (the fluorescence signal was detected during the annealing step). We used the primers FiloAneo: 5′-ARG CMT TYC CAN GYA AYA TGA TGG T-3′ and FiloBNeo: 5′-RTG WGG NGG RYT RTA AWA RTC ACT NAC ATG-3′ and the probe Lloviu-S: FAM-5′-CCT AGA TTG CCC TGT TCA TGA TGC CA-BHQ1-3′16. It is noteworthy that a previously described detection method published by our laboratory15 resulted in a significant number of false positives, which in certain cases we subsequently failed to validate with a pan-filovirus nested-PCR method and sequencing7. All experiments were run on the MyGo Pro PCR system platform (IT-IS Life Science, Ireland) and analysed on the MyGo PCR software (v.3.5.21).
Virus isolation
Cell lines used in this study: Cercopithecus aethiops (African green monkey kidney) cells (Vero E6; ATCC® CRL-1586™), Miniopterus sp. kidney cells (SuBK12-08; kindly provided by Ayato Takada, Hokkaido University)18,35, human neuroblastoma cells (SH-SY5Y; ATCC® CRL-2266™), human hepatocellular carcinoma (Hep G2; ATCC HB-8065™), human non-small cell lung carcinoma (HCC-78; DSMZ ACC 563), human colon carcinoma (HCT 116; DSMZ ACC 581), Tadarida brasiliensis lung cells (Tb 1 Lu; ATCC® CCL-88™).
Vero E6 cells were maintained in Dulbecco’s modified Eagle medium (DMEM, Lonza Cat. No: 12-604 F.), SH-SY5Y and Hep G2 cells were maintained in DMEM with 1% of MEM Non-essential Amino Acid Solution 100× (NEAA) (Sigma Cat. No: M7145), SuBK12-08 and Tb 1 Lu in Eagle’s Minimum Essential Medium (EMEM, Lonza Cat. No: 12-662 F) with 1% of L-Glutamine (200 mM) (Lonza Cat. No: BE17-605E), HCC-78 in RPMI-1640 (Sigma Cat. No: R5886), HCT 116 in McCoy’s 5 A (Lonza, Cat. No: BE12-688F). All culture medium was supplemented with 10% heat inactivated Fetal Bovine Serum (FBS) (Gibco Cat. No: 16140071), and 1% Penicillin/Streptomycin (10,000 U/mL). All cell lines were grown at 37 °C, 5% CO2.
Freshly obtained blood samples from field PCR-tested LLOV RNA-positive bats were frozen in liquid nitrogen and transferred to the BSL-4 laboratory at the Szentágothai Research Center to perform virus isolation experiments. Of note, due to the low body weight of the animals, the blood sample volumes are extremely low, usually below 50 μL. The blood samples (5–50 µL, depending on the original amount) were complemented with cell culture media to a volume of up to 50 μL and used to inoculate cell monolayers seeded in 24-well plates with ~80% confluency. After an 1 h incubation at 37 °C and 5% CO2, the cells were washed once with cell culture media. The cells were monitored daily for cytopathic effects. After 7 days, we performed two freeze-thaw cycles before culture supernatants were transferred to fresh cells. After three blind passages, cells were lysed by the freeze-thaw method, subjected to nucleic acid extraction by Direct-Zol RNA MiniPrep (Zymo Research, USA) and analyzed for the presence of LLOV RNA by RT-PCR using LLOV-specific primers. Viral titers were determined by tissue culture infectious dose (TCID50) assays on SuBK12-08 cells using the Spearman-Karber algorithm.
RNA fluorescence in situ hybridization analysis
For RNA fluorescent in situ hybridization (FISH) analysis, Vero E6 cells seeded in 8-well chamber slides were mock-infected or infected with LLOV at an MOI of 3. Cells were fixed 1 day post-infection in 10% formalin for at least 6 h. RNA FISH was performed using the RNAscope Multiplex Fluorescent V2 kit (Advanced Cell Diagnostics). Viral RNA was detected using custom-designed probes targeting the negative-sense genomic sequence of the LLOV NP gene (Advanced Cell Diagnostics) and stained with Opal 690 fluorophore (Perkin-Elmer). Staining was performed according to the manufacturer’s protocol for adherent cell samples, with the exception of an additional HRP blocking step following signal development of the probes detecting viral mRNA as per the manufacturer’s recommendation. Nuclei were stained with kit- supplied DAPI following the manufacturer’s protocol. Coverslips were mounted on slides using FluorSave mounting medium, and slides were subsequently stored at 4 °C prior to imaging. Images were acquired at ×60 magnification using a Nikon Ti2 Eclipse microscope and Photometrics Prime BSI camera with NIS Elements AR software.
Generation of LLOV pseudotyped lentiviruses and neutralization tests
The LLOV GP gene (GenBank accession JF828358) was inserted into the pCAGGS expression plasmid (kind gift of Prof Ayato Takada). Generation of LLOV and EBOV GP pseudotyped lentiviruses was based on a protocol described previously59, with co-transfection of three plasmids, pCAGGS-LLOV GP or pCAGGS-EBOV GP, p8.91 (HIV-1 gag-pol) and pCSFLW (luciferase reporter), into HEK293/17 cells, using 1 mg/mL branched Polyethyleneimine (PEI) (Sigma-Aldrich, USA) at a ratio of 1:10 (µg DNA: µL PEI). Pseudotyped lentivirus (PV) supernatant was harvested 48 h later and stored at −80 °C. Titration of PVs was carried out as described elsewhere60 by twofold serial dilution across white Nunc flat-bottomed 96-well microplates (Thermo Fisher Scientific, USA) in HEK293T/17 target cells (2 × 104/well), incubated at 37 °C, 5% CO2. After 48 h, the media was removed and discarded; Bright-Glo reagent was added to the plate and incubated at room temperature for 5 min before measuring luminescence on a GloMax 96 luminometer (Promega, USA), with titers given in Relative Light Units (RLU) per mL. For ease of inter-lab comparison, TCID50 titers were also obtained using the same methodology but using a fivefold dilution series.
Luminescence values were used to calculate the PV titre (TCID50/mL) using the Reed-Muench method61. The cumulative number of positive and negative wells for PV infection at each dilution was determined and the percentage calculated for each. The threshold value for a positive well was set at 2.5× the average luminescence value of the cell-only negative controls (Supplementary Fig. 2).
Pseudotyped virus neutralization tests (PVNTs) were performed in white microplates by serial dilution of bat sera (1:40 to 5120; 1:100 to 12,800 or 1:200 to 25,600) with PVs (~1 × 105 RLU/well or ~100 TCID50/well, calculated according to the titration result) incubated at 37 °C for 1 h. Target HEK293/17 cells were then added and plates read was performed60,62. In total, 78 serum samples, mainly from live bats, were tested. Control serum standards (WHO NIBSC15/262 & NIBSC15/282 antibody standards) obtained from EBOV disease convalescent patients were used, as no LLOV-positive serum samples were available. Data was normalized to % reduction in luminescence with respect to the average RLU of cell only (100% neutralization) and PV only (0% neutralization) controls and fitted into a non-linear regression model (log [inhibitor] vs. normalized response, variable slope). IC50 antibody titres were calculated using Prism 8 software. Average values of two independent experiments are indicated unless otherwise stated. The cut-off for PVNT positivity was determined using IC50 values from samples where the total antibody-mediated reduction in RLU was <20% of the PV + no serum control (i.e., 0% neutralization) or when a neutralization of <70% was achieved. The mean of these values was calculated and a cut-off was defined for values higher than the mean + three times the standard deviations63–65 (Supplementary Fig. 5).
Amplicon-based nanopore sequencing
We developed an amplicon sequencing method based on previous protocols66,67 that is able to amplify the LLOV-specific gene regions in two parallel multiplex PCR reactions37. LLOV RNA-positive samples LLOV_378 and LLOV_378P1 were used for sequencing. cDNA preparation from RNA samples was conducted with SuperScript IV (Invitrogen, USA) with random hexamers. Amplicons were generated from cDNA with Q5 Hot Start HF Polymerase (New England Biolabs, USA), with three different primer sets in parallel pools (namely: LLOV_400bp pool 1 and 2, LLOV_500bp pool 1 and 2, LLOV_2000bp pool 1 and 2) (Supplementary Fig. 2). 2.5 µL amplicons from each pool belonging to the same primer set (Primer set: 400 or 500 or 2000) were diluted in 45 µL nuclease-free water before the end repair and dA tailing were performed with the NEBNext Ultra II End Repair/dA-Tailing Module (New England Biolabs, USA). 1.5 µL end-prepped DNA was transferred to the next reaction directly and the barcodes derived from EXP-NBD196 (Oxford Nanopore Technologies, UK) were ligated with NEBNext Ultra II Ligation Module (New England Biolabs, USA). After the clean up of the pooled different barcoded samples jointly with Ampure XP beads (Beckman Coulter, USA), the AMII sequencing adapters were ligated with NEBNext Quick Ligation Module. The final library was quantified with Qubit dsDNA HS Assay Kit (Invitrogen, USA) on a Qubit 3 fluorometer. 25 ng final libraries were loaded onto a R9.4.1 (FLO-MIN106D) flow cell and were sequenced for 48 h37.
Bioinformatic pipeline
The raw sequencing data were basecalled using guppy (ONT guppy v4.4.2.) high accuracy basecaller algorithm (dna_r9.4.1_450bps_hac config file). Demultiplexing and trimming of barcodes were performed also with guppy using default parameters of’guppy_barcoder’ runcode. The ONT guppy software was used under Ubuntu Linux 18.04. Because ARTIC-like protocols generate chimeric reads, we performed length filtering with appropriate lengths specific to the used primer set (LLOV_400bp: 350-600 bp, LLOV_500bp: 440–700 bp, LLOV_2000bp: 1400–2700 bp). Primers were trimmed with the BBDuk (v38.84) Geneious Prime (v2021.1.1) plugin. To generate a consensus sequence, the processed reads from all samples were mapped to the LLOV reference genome (NCBI accession number: NC_016144) with the usage of MiniMap 2.1768. The generated consensus sequences were manually checked for basecalling errors.
Sequencing of the virus isolate
Prior to Nanopore sequencing, virus isolates were exposed to a generally used enrichment protocol69,70. Nucleic acid was extracted from samples using the Direct-zol RNA Miniprep Plus Kit (Zymo Research, USA). Samples were then subjected to the Sequence Independent Single Primer Amplification (SISPA) approach with minor modifications to the previously described protocol70. cDNA amplification was performed by SuperScript IV Reverse Transcriptase (Thermo Fisher scientific) with dNTPs (10 µM) and 2 µM K-8N primer following the manufacturer’s instructions. In order to convert double stranded cDNA from first strand cDNA, samples were subjected to Klenow reaction. Thereafter, the products underwent a purification step using Agencourt AMPure XP beads (Beckman Coulter). Following that, ds cDNA was amplified by Q5® High-Fidelity DNA Polymerase (NEB). Amplified cDNA was purified by Agencourt AMPure XP beads (Beckman Coulter) and quantified using a Qubit dsDNA BR Assay kit (Thermo Fisher Scientific). The double stranded fragments were end prepped with NEBNext Ultra II End Repair/dA-Tailing Module (New England Biolabs, USA) and were barcoded with EXP-NBD196 (Oxford Nanopore Technologies, UK) kit. The final library was sequenced on a R9.4.1 (FLO-MIN106D) flow cell.
The raw sequencing data were basecalled using guppy (ONT guppy v5.0.7.) super-accuracy basecaller algorithm (dna_r9.4.1_450bps_sup config file). Demultiplexing and trimming of barcodes were performed also with guppy using default parameters of’guppy_barcoder’ runcode. The ONT guppy software was used under Ubuntu Linux 18.04. Porechop v0.2.4 was used to trim primer sequences used for SISPA amplification. To generate a consensus sequence, Medaka v1.4.2 was used to map the trimmed reads against the LLOV reference genome (NCBI accession number: NC_016144) and perform variant calling. Extension of the genome ends was done by manually filling in the observed overhangs based on a mapping performed using the Long Read Support tool in CLC Genomics workbench v20.0.4. Extension was stopped when read coverage fell below 20×.
Phylogenetic tree caption
Prior to the phylogenetic tree implementation, 22 full length sequences belonging to the family Filoviridae were aligned in the MAFFT webserver (version 7 https://mafft.cbrc.jp/alignment/server/) using default parameters. Subsequently, a best substitution model selection was performed in IQ-tree webserver (http://iqtree.cibiv.univie.ac.at/) and thus a Bayesian phylogeny tree was generated using BEAST v1.10.471 under a GTR + G + I substitution model, assuming a constant population size and a strict molecular clock (uniform rates across branches). The MCMC chains were run for 10,000,000 iterations and sampled each 1,000th generation. The effective sampling size was checked in Tracer software (>200). Thereafter, the Maximum clade credibility tree (MCC) was generated using TreeAnnotator and edited in the iTol webserver (https://itol.embl.de/).
Statistics and reproducibility
Depending on the available amount of bat sera different measurement and dilution strategies were applied: measured as duplicate with repeated experiment—bat ♯1, ♯169, ♯170, ♯99, ♯115, ♯130; measured in duplicate with single experiment—bat ♯2, ♯102, ♯138; measured in single in one experiment—bat ♯98, ♯110, ♯118, ♯143. All dilutions, including standard sera were 1/40, 1/80, 1/160, 1/320, 1/640, 1/1280, 1/2560, 1/5120 except for bat ♯143 where 1/100 starting dilution was applied.
In vitro isolation experiments were performed in triplicates with same results. The RNA FISH analysis was performed once. The probes were evaluated with recombinant LLOV and reliably detected positive and negative-sense recombinant LLOV RNA in infected cells21.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
The project was supported by the National Research, Development and Innovation Office (grant numbers: NKFIH FK131465 (G.K.), KH129599 (G.K., F.J.). G.K. was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences. G.E.T. and Z.L. were supported by the Biological and Sportbiological Doctoral School of the University of Pécs, Hungary. The SuBK12-08 cell line was obtained from the scientists of Hokkaido University Center for Zoonosis Control and the University of Zambia. The authors wish to thank professor Ayato Takada for sharing the SuBK12-08 cells. This work was supported by NIH grants R21 AI137793 (E.M.) and R01 AI133486 (E.M.). E.L.S. was supported by NIH training grant T32 HL007035.
Source data
Author contributions
G.K., G.E.T., M.M.N., S.S., B.Z., Z.L., Á.N, C.I.P., G.D., F.F. conceived the laboratory work. G.K., G.E.T., S.A.B., T.G., P.E., Z.L., Á.N., C.I.P., G.D., K.K. conducted field work. M.M.N., S.S., N.T., E.W. designed and conducted the neutralization experiments. S.A.B., T.G., P.E. handling of bats during field work. P.E., T.S. ectoparasite identification. G.K., M.M., F.F. conceived the in vitro isolation experiments. E.L.S. performed the RNA FISH experiments. G.E.T., B.A.S., A.J.H., S.Z., G.K., P.M, B.V. sequencing and genomic analysis. B.A.S., M.M.N. visualization of results. G.K., F.J., S.S. conceptualization and supervision. G.K. wrote the paper. G.E.T., M.M.N., S.S., N.T., E.W., S.A.B., T.G., Á.N., F.J., A.J.H., E.M. edited the paper with contributions from all other authors. G.K., S.S., E.M., Á.N., F.J. provided supervision. All authors approved the paper.
Peer review
Peer review information
Nature Communications thanks Arinjay Banerjee and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Funding
Open access funding provided by University of Pécs.
Data availability
The genomic sequence data in this study have been deposited in the NCBI GenBank database under accession codes: MW775010, MW775011 and MZ541881. Tick samples: OL795929-OL795963 [https://www.ncbi.nlm.nih.gov/nuccore/?term=ixodes+lloviu+hungary]. The background data of sampled animals, such as sex and collection dates are provided in the Supplementary Data 1. Sequencing protocol and materials are listed here: https://www.protocols.io/view/lloviu-cuevavirus-sequencing-protocol-bmz3k78n.html. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Gábor Kemenesi, Gábor Endre Tóth.
Change history
9/6/2022
A Correction to this paper has been published: 10.1038/s41467-022-32735-w
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-29298-1.
References
- 1.Burk R, et al. Neglected filoviruses. FEMS Microbiol. Rev. 2016;40:494–519. doi: 10.1093/femsre/fuw010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malvy D, McElroy AK, de Clerck H, Günther S, van Griensven J. Ebola virus disease. Lancet. 2019;393:936–948. doi: 10.1016/S0140-6736(18)33132-5. [DOI] [PubMed] [Google Scholar]
- 3.Jombart T, et al. The cost of insecurity: From flare-up to control of a major Ebola virus disease hotspot during the outbreak in the Democratic Republic of the Congo, 2019. Eurosurveillance. 2020;25:1–4. doi: 10.2807/1560-7917.ES.2020.25.2.1900735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebola virus disease outbreak in Guinea. https://www.ecdc.europa.eu/en/publications-data/ebola-virus-disease-outbreak-guinea-2021 (2021).
- 5.Leroy EM, et al. Fruit bats as reservoirs of Ebola virus. Nature. 2005;438:575–576. doi: 10.1038/438575a. [DOI] [PubMed] [Google Scholar]
- 6.Towner JS, et al. Isolation of genetically diverse Marburg viruses from Egyptian fruit bats. PLoS Pathog. 2009;5:e1000536. doi: 10.1371/journal.ppat.1000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He B, et al. Filovirus RNA in Fruit Bats, China. Emerg. Infect. Dis. 2015;21:1675–1677. doi: 10.3201/eid2109.150260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X-L, et al. Genetically Diverse Filoviruses in Rousettus and Eonycteris spp. Bats, China, 2009 and 2015. Emerg. Infect. Dis. 2017;23:482–486. doi: 10.3201/eid2303.161119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi M, et al. The evolutionary history of vertebrate RNA viruses. Nature. 2018;556:197–202. doi: 10.1038/s41586-018-0012-7. [DOI] [PubMed] [Google Scholar]
- 10.Yang XLou, et al. Characterization of a filovirus (Měnglà virus) from Rousettus bats in China. Nat. Microbiol. 2019;4:390–395. doi: 10.1038/s41564-018-0328-y. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein T, et al. The discovery of Bombali virus adds further support for bats as hosts of ebolaviruses. Nat. Microbiol. 2018;3:1084–1089. doi: 10.1038/s41564-018-0227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forbes KM, et al. Bombali Virus in Mops condylurus Bat, Kenya. Emerg. Infect. Dis. 2019;25:955–957. doi: 10.3201/eid2505.181666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karan LS, et al. Bombali virus in Mops condylurus bats, Guinea. Emerg. Infect. Dis. 2019;25:1774–1775. doi: 10.3201/eid2509.190581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Negredo A, et al. Discovery of an ebolavirus-like filovirus in europe. PLoS Pathog. 2011;7:e1002304. doi: 10.1371/journal.ppat.1002304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kemenesi G, et al. Re-emergence of Lloviu virus in Miniopterus schreibersii bats, Hungary, 2016 correspondence. Emerg. Microbes Infect. 2018;7:1–4. doi: 10.1038/s41426-018-0067-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Arellano ER, et al. First evidence of antibodies against lloviu virus in schreiber’s bent-winged insectivorous bats demonstrate a wide circulation of the virus in spain. Viruses. 2019;11:360. doi: 10.3390/v11040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brinkmann C, et al. The tetherin antagonism of the Ebola virus glycoprotein requires an intact receptor-binding domain and can be blocked by GP1-specific antibodies. J. Virol. 2016;90:11075–11086. doi: 10.1128/JVI.01563-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maruyama J, et al. Characterization of the envelope glycoprotein of a Novel Filovirus, Lloviu Virus. J. Virol. 2014;88:99–109. doi: 10.1128/JVI.02265-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng M, et al. Cell entry by a novel European filovirus requires host endosomal cysteine proteases and Niemann–Pick {C1} Virology. 2014;468–470:637–646. doi: 10.1016/j.virol.2014.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manhart WA, et al. A Chimeric Lloviu virus minigenome system reveals that the bat-derived filovirus replicates more similarly to ebolaviruses than marburgviruses. Cell Rep. 2018;24:2573–2580.e4. doi: 10.1016/j.celrep.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hume AJ, et al. Recombinant Lloviu virus as a tool to study viral replication and host responses. PLoS Pathog. 2021;4:e1010268. doi: 10.1371/journal.ppat.1010268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gryseels S, et al. Role of Wildlife in Emergence of Ebola Virus in Kaigbono (Likati), Democratic Republic of the Congo, 2017. Emerg. Infect. Dis. 2020;26:2205–2209. doi: 10.3201/eid2609.191552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amman BR, et al. Seasonal Pulses of Marburg Virus circulation in Juvenile Rousettus aegyptiacus Bats Coincide with Periods of Increased Risk of Human Infection. PLoS Pathog. 2012;8:e1002877. doi: 10.1371/journal.ppat.1002877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayman DTS. Biannual birth pulses allow filoviruses to persist in bat populations. Proc. Biol. Sci. 2015;282:20142591. doi: 10.1098/rspb.2014.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breman JG, et al. A search for Ebola virus in animals in the Democratic Republic of the Congo and Cameroon: ecologic, virologic, and serologic surveys, 1979-1980. Ebola Virus Study Teams. J. Infect. Dis. 1999;179:S139–S147. doi: 10.1086/514278. [DOI] [PubMed] [Google Scholar]
- 26.Leirs H, et al. Search for the Ebola Virus Reservoir in Kikwit, Democratic Republic of the Congo: Reflections on a Vertebrate Collection. J. Infect. Dis. 1999;179:S155–S163. doi: 10.1086/514299. [DOI] [PubMed] [Google Scholar]
- 27.Reiter P, et al. Field Investigations of an Outbreak of Ebola Hemorrhagic Fever, Kikwit, Democratic Republic of the Congo, 1995: Arthropod Studies. J. Infect. Dis. 1999;179:S148–S154. doi: 10.1086/514304. [DOI] [PubMed] [Google Scholar]
- 28.Szentiványi T, Christe P, Glaizot O. Bat flies and their microparasites: current knowledge and distribution. Front. Vet. Sci. 2019;6:115. doi: 10.3389/fvets.2019.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dick, C. W. & Patterson, B. D. Bat flies: Obligate ectoparasites of bats. In Micromammals and Macroparasites: From Evolutionary Ecology to Management 179–194 (Springer Japan, 2006). 10.1007/978-4-431-36025-4_11.
- 30.Reeves, W. K. & Lloyd, J. E. Louse flies, keds, and bat flies (hippoboscoidea). in Medical and Veterinary Entomology 421–438 (Elsevier, 2018). 10.1016/B978-0-12-814043-7.00020-0.
- 31.Szentiványi T, Estók P, Földvári M. Checklist of host associations of European bat flies (Diptera: Nycteribiidae, Streblidae) Zootaxa. 2016;4205:101–126. doi: 10.11646/zootaxa.4205.2.1. [DOI] [PubMed] [Google Scholar]
- 32.Quick J, et al. Real-time, portable genome sequencing for Ebola surveillance. Nature. 2016;530:228–232. doi: 10.1038/nature16996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quick J, et al. Multiplex PCR method for MinION and Illumina sequencing of Zika and other virus genomes directly from clinical samples. Nat. Protoc. 2017;12:1261–1266. doi: 10.1038/nprot.2017.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuh AJ, et al. Comparative analysis of serologic cross-reactivity using convalescent sera from filovirus-experimentally infected fruit bats. Sci. Rep. 2019;9:1–12. doi: 10.1038/s41598-019-43156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Y T, et al. Niemann-Pick C1 heterogeneity of bat cells controls filovirus tropism. Cell Rep. 2020;30:308–319.e5. doi: 10.1016/j.celrep.2019.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tyson, J. R. et al. Improvements to the ARTIC multiplex PCR method for SARS-CoV-2 genome sequencing using nanopore. bioRxiv3, 1 10.1101/2020.09.04.283077 (2020).
- 37.Lloviu cuevavirus sequencing protocol. https://www.protocols.io/view/lloviu-cuevavirus-sequencing-protocol-bmz3k78n.html. (2020).
- 38.Mandl JN, et al. Reservoir host immune responses to emerging zoonotic viruses. Cell. 2015;160:20–35. doi: 10.1016/j.cell.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones MEB, et al. Clinical, histopathologic, and immunohistochemical characterization of experimental marburg virus infection in a natural reservoir host, the Egyptian Rousette bat (Rousettus aegyptiacus) Viruses. 2019;11:214. doi: 10.3390/v11030214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caron A, et al. Ebola virus maintenance: if not (Only) bats, what else? Viruses. 2018;10:549. doi: 10.3390/v10100549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayman DTS. As the bat flies. Science. 2016;354:1099–1100. doi: 10.1126/science.aaj1818. [DOI] [PubMed] [Google Scholar]
- 42.Kohl C, Nitsche A, Kurth A. Update on potentially Zoonotic viruses of European bats. Vaccines. 2021;9:690. doi: 10.3390/vaccines9070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Natesan M, et al. Human survivors of disease outbreaks caused by Ebola or Marburg virus exhibit cross-reactive and long-lived antibody responses. Clin. Vaccin. Immunol. 2016;23:717–724. doi: 10.1128/CVI.00107-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kamata T, Natesan M, Warfield K, Aman MJ, Ulrich RG. Determination of specific antibody responses to the six species of Ebola and Marburg viruses by multiplexed protein microarrays. Clin. Vaccin. Immunol. 2014;21:1605. doi: 10.1128/CVI.00484-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amman BR, et al. Marburgvirus resurgence in Kitaka mine bat population after extermination attempts, Uganda. Emerg. Infect. Dis. 2014;20:1761–1764. doi: 10.3201/eid2010.140696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Subudhi S, Rapin N, Misra V, et al. Immune system modulation and viral persistence in bats: understanding viral spillover. Viruses. 2019;11:192. doi: 10.3390/v11020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pawęska JT, Jansen van Vuren P, Storm N, Markotter W, Kemp A. Vector competence of Eucampsipoda africana (Diptera: Nycteribiidae) for Marburg virus transmission in Rousettus aegyptiacus (Chiroptera: Pteropodidae) Viruses. 2021;13:2226. doi: 10.3390/v13112226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lourenço S, Palmeirim JM. Which factors regulate the reproduction of ectoparasites of temperate-zone cave-dwelling bats? Parasitol. Res. 2008;104:127–134. doi: 10.1007/s00436-008-1170-6. [DOI] [PubMed] [Google Scholar]
- 49.Turell MJ, Bressler DS, Rossi CA. Short report: Lack of virus replication in arthropods after intrathoracic inoculation of Ebola Reston virus. Am. J. Trop. Med. Hyg. 1996;55:89–90. doi: 10.4269/ajtmh.1996.55.89. [DOI] [PubMed] [Google Scholar]
- 50.Schuh AJ, et al. No evidence for the involvement of the argasid tick Ornithodoros faini in the enzootic maintenance of marburgvirus within Egyptian rousette bats Rousettus aegyptiacus. Parasites Vectors. 2016;9:128. doi: 10.1186/s13071-016-1390-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Szentiványi T, et al. Host conservation through their parasites: molecular surveillance of vector-borne microorganisms in bats using ectoparasitic bat flies. Parasite. 2020;27:72. doi: 10.1051/parasite/2020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jun SR, et al. Ebolavirus comparative genomics. FEMS Microbiol. Rev. 2015;39:764–778. doi: 10.1093/femsre/fuv031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Towner JS, et al. Marburgvirus genomics and association with a large hemorrhagic fever outbreak in Angola. J. Virol. 2006;80:6497–6516. doi: 10.1128/JVI.00069-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dietz, C. Illustrated Identification Key to the Bats of Europe. Electronical Publication, Version 1.0 https://www.researchgate.net/publication/274838308_llustrated_Identification_key_to_the_Bats_of_Europe_-_complete_pdf (2004).
- 55.Traub R, et al. Catalogue of Nycteribiids. Nature. 1967;216:725. [Google Scholar]
- 56.HILLYARD, P. D. Ticks of North-west Europe. (Field Studies Council, for the Linnean Society of London and the Estuarine and Coastal Sciences Association, 1996).
- 57.Lv J, et al. Assessment of four DNA fragments (COI, 16S rDNA, ITS2, 12S rDNA) for species identification of the Ixodida (Acari: Ixodida) Parasit. Vectors. 2014;7:93. doi: 10.1186/1756-3305-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He B, et al. Identification of a novel Orthohepadnavirus in pomona roundleaf bats in China. Arch. Virol. 2015;160:335–337. doi: 10.1007/s00705-014-2222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mather ST, Wright E, Scott SD, Temperton NJ. Lyophilisation of influenza, rabies and Marburg lentiviral pseudotype viruses for the development and distribution of a neutralisation—assay-based diagnostic kit. J. Virol. Methods. 2014;210:51–58. doi: 10.1016/j.jviromet.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 60.Scott S, Molesti E, Temperton N. The use of equine influenza pseudotypes for serological screening. J. Mol. Genet. Med. 2012;06:304–308. [PMC free article] [PubMed] [Google Scholar]
- 61.Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints12. Am. J. Epidemiol. 1938;27:493–497. [Google Scholar]
- 62.Ferrara F, Temperton N. Pseudotype neutralization assays: from laboratory bench to data analysis. Methods Protoc. 2018;1:1–16. doi: 10.3390/mps1010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jacobson RH, et al. Validation of serological assays for diagnosis of infectious diseases. Rev. Sci. Tech. Off. Int. Epiz. 1998;17:469–526. doi: 10.20506/rst.17.2.1119. [DOI] [PubMed] [Google Scholar]
- 64.Lester S, et al. Middle East respiratory coronavirus (MERS-CoV) spike (S) protein vesicular stomatitis virus pseudoparticle neutralization assays offer a reliable alternative to the conventional neutralization assay in human seroepidemiological studies. Access Microbiol. 2019;1:e000057. doi: 10.1099/acmi.0.000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nie J, et al. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. Emerg. Microbes Infect. 2020;9:680–686. doi: 10.1080/22221751.2020.1743767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Quick, J. Ebola virus sequencing protocol. 10.17504/protocols.io.7nwhmfe (2019).
- 67.Quick, J. Forked from Ebola virus sequencing protocol. 10.17504/protocols.io.bbmuik6w (2020).
- 68.Li H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics. 2018;34:3094–3100. doi: 10.1093/bioinformatics/bty191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Conceiacao-Neto N. et al. Modular approach to customise sample preparation procedures for viral metagenomics: a reproducible protocol for virome analysis. Sci. Rep. 5 (2015). [DOI] [PMC free article] [PubMed]
- 70.Chrzastek K, et al. Use of Sequence-Independent, Single-Primer-Amplification (SISPA) for rapid detection, identification, and characterization of avian RNA viruses. Virology. 2017;509:159–166. doi: 10.1016/j.virol.2017.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suchard MA, et al. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018;4:vey016. doi: 10.1093/ve/vey016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The genomic sequence data in this study have been deposited in the NCBI GenBank database under accession codes: MW775010, MW775011 and MZ541881. Tick samples: OL795929-OL795963 [https://www.ncbi.nlm.nih.gov/nuccore/?term=ixodes+lloviu+hungary]. The background data of sampled animals, such as sex and collection dates are provided in the Supplementary Data 1. Sequencing protocol and materials are listed here: https://www.protocols.io/view/lloviu-cuevavirus-sequencing-protocol-bmz3k78n.html. Source data are provided with this paper.