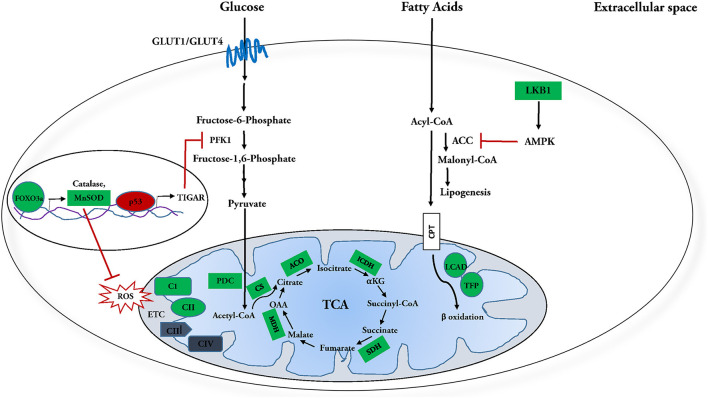
Figure 1.
Molecular targets of SIRT3 in glucose and fatty acid metabolism. SIRT3 and fatty acid metabolism: SIRT3 deacetylates and activates enzymes involved in fatty acid oxidation, including long chain aycl-CoA dehydrogenase (LCAD) and trifunctional mitochondrial protein (TFP). Meanwhile it inhibits fatty acid synthesis by deacetylating and activating its inhibitor, LKB1, which in turn activates AMPK. AMPK further phosphorylates and inhibits Acetyl-CoA carboxylase (ACC) and Malonyl-CoA decarboxylase (MCD), reducing synthesis of malonyl-CoA, a negative regulator of fatty acid oxidation. In this manner, SIRT3 regulation culminates in enhanced fatty acid catabolism. SIRT3 and glucose metabolism: SIRT3 attenuates-activation of FOXO3a, which in turn transcriptionally upregulates ROS detoxification enzymes manganese dependent super oxide dismutase (MnSOD) and catalase. SIRT3 also directly interacts with and activates MnSOD. Attenuation of ROS inhibits HIF-1α from upregulating glycolytic genes during normoxia. SIRT3 enhances phosphofructokinase 1 (PFK 1) activity and subsequently upregulates glycolysis by increasing PFKFB3 activity via deacetylation-inactivation of p53 and subsequent suppression of its downstream target TIGAR. SIRT3 further increases glucose oxidation by activating pyruvate dehydrogenase complex (PDC) and targeting the enzymes involved in the tricarboxylic acid (TCA) cycle. SIRT3 mediated deacetylation of complex I subunit, NDUFA9 and succinate dehydrogenase (SDH) is necessary for efficient oxidative phosphorylation. In this manner, SIRT3 regulation culminates in enhanced utilization of glucose. Positively regulated SIRT3 molecular targets are indicated in green; negatively regulated targets are indicated in red.