Abstract
STUDY QUESTION
Is periconceptional maternal smoking associated with embryonic morphological development in ongoing pregnancies?
SUMMARY ANSWER
Smoking during the periconceptional period is associated with a delayed embryonic morphological development which is not fully recuperated beyond the first trimester of pregnancy.
WHAT IS KNOWN ALREADY
Smoking during pregnancy decreases prenatal growth, increasing the risk of preterm birth, small for gestational age (GA) and childhood obesity.
STUDY DESIGN, SIZE, DURATION
Between 2010 and 2018, 689 women with ongoing singleton pregnancies were periconceptionally enrolled in a prospective cohort study with follow-up until 1 year after delivery.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Between 7 + 0 and 10 + 3 weeks, GA serial three-dimensional transvaginal ultrasound scans were performed. Embryonic morphological development as assessed by the Carnegie developmental stages was evaluated using Virtual Reality techniques. In the absence of fetal morphology classification methods beyond the embryonic period, fetal ultrasound measurements at around 20 weeks’ GA, and birth weight were used to assess fetal growth. Linear mixed models were used to evaluate the association between smoking and the Carnegie stages. Regarding first-trimester morphological development, we additionally stratified our findings for mode of conception. Multiple linear regression models were used to study the association between smoking, fetal growth and birth weight. To investigate to which extent delayed embryonic morphological development mediated the effect of smoking, contemporary mediation analysis was used. Adjustments were made for potential confounders and other covariates.
MAIN RESULTS AND THE ROLE OF CHANCE
A total of 689 singleton ongoing pregnancies were included and 1210 Carnegie stages were determined. Maternal periconceptional smoking represented by the number of cigarettes/day was associated with a slight non-significant delay of the Carnegie stages (βcigarettes/day = −0.058, 95% CI −0.122; 0.007, P = 0.080). Smoking of ≥10 cigarettes/day showed the strongest association (β≥10 cigarettes/day = −0.352, 95% CI −0.648; −0.057, P = 0.019), as reflected by a 0.9-day delay in reaching the final Carnegie stage. Stratification for mode of conception showed a stronger negative association between the number of cigarettes/day in the IVF/ICSI group (βcigarettes/day = −0.126, 95% CI −0.200; −0.051, P = 0.001) compared to naturally conceived pregnancies (βcigarettes/day = 0.009, 95% CI −0.093; 0.111, P = 0.867). In the IVF/ICSI group, periconceptional smoking of ≥10 cigarettes/day was associated with in a 1.6 day delay in reaching the final Carnegie stage (β≥10 cigarettes/day = −0.510, 95% CI −0.834; −0.186, P = 0.002). In the second trimester, periconceptional smoking was associated with a smaller femur length (βcigarettes/day = −0.077, 95% CI −0.147; −0.008, P = 0.029) and a larger head circumference (β1–9 cigarettes/day = 0.290, 95% CI 0.065; 0.514, P = 0.012). Smoking was associated with a lower birth weight, with a dose-response effect (βcigarettes/day = −0.150, 95% CI −0.233; −0.068, P < 0.001). Furthermore, using the unadjusted model, 40–60% of the association between smoking and fetal ultrasound parameters and 6.3% of the association between smoking and birth weight can be explained by a delayed embryonic morphology.
LIMITATIONS, REASONS FOR CAUTION
The study population was recruited from a tertiary referral center. Smoking habits were explored using self-reported questionnaires and checked for consistency by trained researchers.
WIDER IMPLICATIONS OF THE FINDINGS
This study shows that the association of periconceptional maternal smoking and human morphological development can already be detected early in the first trimester of pregnancy using embryonic morphology as outcome. One of the key messages of this study is that the delay, or dysregulation, in embryonic morphology is associated with allometric growth reflected by smaller fetal measurements at 20 weeks gestation and lower weight at birth. The delay in embryonic morphology, measured in early pregnancy, cannot be recuperated during the pregnancy. The results of this study emphasize the importance of smoking intervention programs prior to conception. More research is warranted to assess the association between periconceptional smoking cessation and embryonic development.
STUDY FUNDING/COMPETING INTEREST(S)
The work was funded by the Department of Obstetrics and Gynaecology, Erasmus MC, University Medical Centre, Rotterdam, The Netherlands. The authors declare no conflicts of interest.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: embryonic growth, morphology, Carnegie stage, smoking, virtual reality, three-dimensional ultrasound
Introduction
Worldwide, over 2.3 million women per year smoke during pregnancy, resulting in congenital anomalies, premature birth, low birth weight, stillbirth and childhood obesity (Hackshaw et al., 2011; Wagijo et al., 2017; Lange et al., 2018; Kataria et al., 2019; United Nations Department of Economic and Social Affairs Population Division, 2019). To prevent these smoking-related adverse perinatal outcomes, the World Health Organization (WHO) stimulated tobacco control policies and smoke-free legislation (WHO, 2019). Despite tobacco control policies, more than 50% of women continue smoking during pregnancy (Lange et al., 2018). It is unclear whether smoking in the periconceptional period, i.e. a time window of 14 weeks before and up to 10 weeks after conception, already affects early pregnancy development (van Uitert et al., 2013). Considering an effect of tobacco smoke on early pregnancy development, it is crucial to gain an insight into these effects of tobacco smoke on development as the results may benefit patient education and societal smoking policies and ultimately long-term health outcome.
In early pregnancy, embryonic development can be studied by ultrasound. Historically, development was studied by measuring the embryonic crown-rump length (CRL), a two-dimensional (2D) growth parameter. However, assessment of embryonic development should not only be based on embryonic size, it should also include the development of embryonic organs and structures (O'Rahilly and Muller, 2010). Furthermore, the assessment of embryonic development should be independent of gestational age (GA) because of subtle differences in embryonic growth trajectories. The Carnegie developmental stages offer a staging system for embryonic morphological development, independent of GA or growth measurements and based on structural examination. The 23 Carnegie developmental stages are based on postmortem examination of internal and external morphological features of the embryo, such as the development and position of the limbs and curvature of the embryo (O'Rahilly and Muller, 2010). With state-of-the-art imaging techniques such as Virtual Reality (VR), the Carnegie stages can now be assigned in utero (Blaas et al., 1998; Rousian et al., 2018). VR in combination with high-resolution three-dimensional (3D) transvaginal ultrasound allows true depth perception required to evaluate the Carnegie stages of ongoing pregnancies.
Although data on the relationship between prenatal growth and smoking are abundant, it remains unclear whether and during which stage of gestation, embryonic morphology and fetal growth is affected by smoking. Therefore, we hypothesize that embryonic development and fetal growth differs between smoking and non-smoking women. Hence, the aim of this study is to assess associations between maternal smoking in the periconceptional period and embryonic development as reflected by morphology classified according to the Carnegie stages. Classification of fetal morphology beyond the embryonic period is absent. Therefore, we will assess the associations between periconceptional maternal smoking and fetal growth representing development beyond the first trimester.
Materials and methods
This study was embedded in the Rotterdam Periconception Cohort (Predict study), an ongoing prospective study, performed at the Department of Obstetrics and Gynecology of the Erasmus MC, University Medical Center, a tertiary care hospital for preconception and prenatal care in Rotterdam, the Netherlands. The aim of the Predict study was to assess periconceptional determinants and predictors of pregnancy outcome and offspring health and to investigate underlying biological (epigenetic) mechanisms (Steegers-Theunissen et al., 2016).
Data collection
All women attending the outpatient clinic for preconception or prenatal care with an age of ≥18 years and an ongoing pregnancy before 8 weeks GA were eligible for inclusion. Participants in this study were recruited between 2010 and 2018. The included women conceived naturally or through ART. Exclusion criteria were oocyte donation, twin pregnancies, termination of pregnancy and pregnancies with the following adverse outcomes: ectopic implantation, congenital anomaly, miscarriage, fetal death or demise, stillbirth or neonatal death. In addition, pregnancies without available 3D ultrasound examinations were excluded.
Pregnancy dating
GA in naturally conceived pregnancies was calculated from the first day of the last menstrual period (LMP). Pregnancies were excluded in case of a self-reported irregular cycle (<21 or >35 days) or unknown LMP or if the GA based on LMP differed more than 6 days from GA based on CRL. Pregnancies conceived through IUI, artificial insemination with donor sperm or by means of hormone treatment, were classified in the subgroup of naturally conceived pregnancies. In case of IUI or donor insemination, GA was calculated using the insemination date. In IVF and ICSI conceived pregnancies after fresh embryo transfer (ET), GA was calculated from the oocyte retrieval day plus 14 days. In IVF or ICSI pregnancies after frozen-thawed ET, GA was calculated from the day of ET plus 19 days. The calculation of the GA of cryopreserved ETs depended on the number of days between oocyte retrieval and embryonic cryopreservation.
Ultrasound measurements
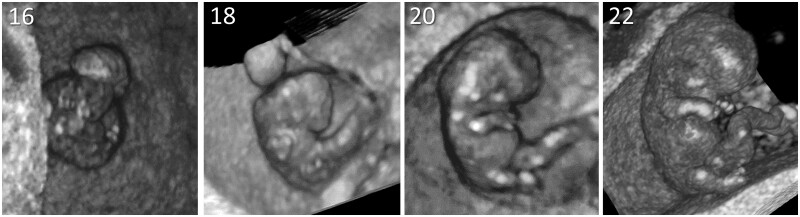
All women received serial transvaginal 3D ultrasound examinations performed by trained examiners using a 6–12 MHz high-resolution probe of a GE Voluson E8 ultrasound machine (GE, Zipf, Austria), adhering to the ‘as low as reasonably achievable’-principle. From 2010 to 2013, weekly scans were performed between 6 and 12 weeks’ GA. After 2013, 3D ultrasound examinations were performed at 7, 9 and 11 weeks’ gestation (van Uitert et al., 2013). All 3D ultrasound data were transformed into Cartesian (rectangular) volumes and examined using the BARCO I-Space. The BARCO I-Space is a VR room, in which V-scope software is used for rendering of holograms and generating real depth perception (Rousian et al., 2018). With the use of VR, the CRL was measured and external and internal morphological features of the embryo were applied by trained researchers (Rousian et al., 2018). The morphological development was visually compared and evaluated using the Carnegie stages 12–23, as described by O'Rahilly and Muller (2010). The assignment of a Carnegie stage is based on the development of the upper or lower limp bud, the positioning of the limb, the development of a hand or foot and the straightening of the trunk. Furthermore, the Carnegie stage is determined by the development of internal structures reflected by morphological features of the pros-, mes- and rhombencephalon (Rousian et al., 2013). Figure 1 shows four embryos reflecting four different Carnegie stages (i.e. stages 16, 18, 20 and 22).
Figure 1.
Three-dimensional virtual reality images of four embryos reflecting four different Carnegie stages. The 23 Carnegie developmental stages are based on examination of internal and external morphological features of the embryo, such as the development and position of the limbs and curvature of the embryo.
Data on second-trimester fetal growth, represented by measurements of head circumference, abdominal circumference and femur length, were obtained from medical records. Between 18 and 22 weeks GA, fetal growth assessment was performed as part of the screening for congenital anomalies. These scans are performed by trained sonographers using a standardized protocol (Dutch Society for Obstetrics and Gynecology, 2019).
Questionnaires
At enrollment, all participants filled out a self-administered general questionnaire on maternal characteristics, providing details of age, ethnic background, educational level (according to CBS-classification (Centraal Bureau voor Statistiek, 2016)), medical and obstetric history, BMI and lifestyle behaviors (i.e. smoking, alcohol consumption, folic acid supplement use). During the intake visit at the outpatient clinic, experienced researchers and research nurses check the questionnaires in a standardized manner for completeness and consistency (Steegers-Theunissen et al., 2016). Smoking was assessed by asking every participant about smoking habits during the period 4 weeks prior to conception and during pregnancy. If the woman did smoke during this timeframe, the participant was asked how many cigarettes or other tobacco products she smoked, expressed as number per day. If the woman had quit smoking, the date of cessation was asked.
General data
At enrollment blood pressure, weight and height were measured and at each subsequent prenatal visit, blood pressure was measured. Trained researchers registered the anthropometric measurements. Data on the infant’s birth outcomes, including GA at delivery and birth weight, were obtained from medical records. Small for GA (SGA) is defined as a birth weight under the tenth percentile for GA (Zeve et al., 2016).
Statistical analysis
Baseline characteristics, second-trimester growth parameters and birth weight of the infants of smoking women were compared to non-smoking women using Students’ t-test or Mann–Whitney U-test for continuous variables and Chi-square or Fisher’s exact test for categorical variables, respectively. The Carnegie stages were treated as a continuous variable with a maximum value of 23 (O'Rahilly and Muller, 2010). Smoking was considered as both a dichotomous (presence/absence) and continuous (number cigarettes/day) variable. A root transformation on smoking as a continuous variable was performed to address an effect of outliers. Finally, smoking was categorized in a dose–response sense into three subgroups: no cigarettes, 1–9 cigarettes/day and 10 or more cigarettes/day in the periconceptional period (van Uitert et al., 2013).
Linear mixed models were used to evaluate the association between smoking and Carnegie stages. At first, a model analysis with GA and maternal smoking as predictors was performed (Model 1). Finally, the complete model (Model 2) also included all potential confounders identified in previous literature (i.e. parity, ethnicity, educational level, alcohol use, folic acid supplement use, multivitamin supplement use, fetal sex, maternal age, BMI and mode of conception) (van Uitert et al., 2013; Rousian et al., 2018). All confounders were entered simultaneously into the model together with a random intercept. Since embryonic growth differs according to the mode of conception, a subgroup analysis with stratification for mode of conception was performed (Berntsen et al., 2019). Furthermore, a subgroup analysis with stratification for fetal sex was performed. Possible interaction between embryonic morphology and fetal sex was examined using a linear mixed model with interaction variables for fetal sex, GA and maternal smoking. To calculate the delay in Carnegie stages expressed in days of gestation, a linear mixed model with interaction variables for smoking and GA was used, also adjusting for potential confounders and covariates.
Multiple linear regression models were used to study the association between smoking and fetal growth parameters in the second trimester and birth weight. Fetal growth parameters were corrected for GA using the INTERGROWTH-21st project growth standards (Papageorghiou et al., 2014). Birth weight was adjusted for GA and sex (Hoftiezer et al., 2019). Second-trimester growth parameters and birth weight were transformed into Z-scores. Model 1 included the variables for maternal smoking and GA. In the fully adjusted model, Model 2, all potential confounders and covariates were entered.
The association between embryonic morphology, maternal periconceptional smoking, fetal growth parameters and birth weight were measured by linear regression. Embryonic morphology at 72 days GA, as a mediator, was entered in a linear regression model with smoking as a continuous and as a categorical variable with fetal growth parameters and birth weight as outcomes. Again, Model 1 included the variables for maternal smoking, GA and the mediator. Model 2, the fully adjusted model, included the crude model and all potential confounders and covariates. The total effect of maternal periconceptional smoking was decomposed into a part mediated by embryonic morphology (corresponding to the indirect effect) and a part mediated by other pathways (corresponding to the direct effect) (Farland et al., 2020). First, interactions between smoking and embryonic morphology on the association between fetal growth parameters and birth weight were examined, but no interactions were found (Pinteraction = 0.544 for head circumference, Pinteraction = 0.694 for abdominal circumference, Pinteraction = 0.183 for femur length and Pinteraction = 0.583 for birth weight). This simplifies the estimation of direct and indirect effects (VanderWeele, 2016). The part of the association between smoking and fetal growth or birth weight that is mediated by embryonic morphology can therefore be estimated as the difference between the total effect and the effect in a model additionally adjusting for embryonic morphology (the indirect effect) (VanderWeele, 2016). The mediated proportion was calculated as the effect mediated by embryonic morphology/total effect. Bootstrapping with 5000 replications was used to compute a 95% CI.
All statistical analysis within this study was performed using IBM SPSS Statistics for Windows, version 25.0 and R studio version 3.6.1. P < 0.05 was considered to indicate statistical significance.
Ethical approval
The protocol had been approved by the local medical ethics committee and all women signed a written informed consent before participation, also on behalf of their unborn child.
Results
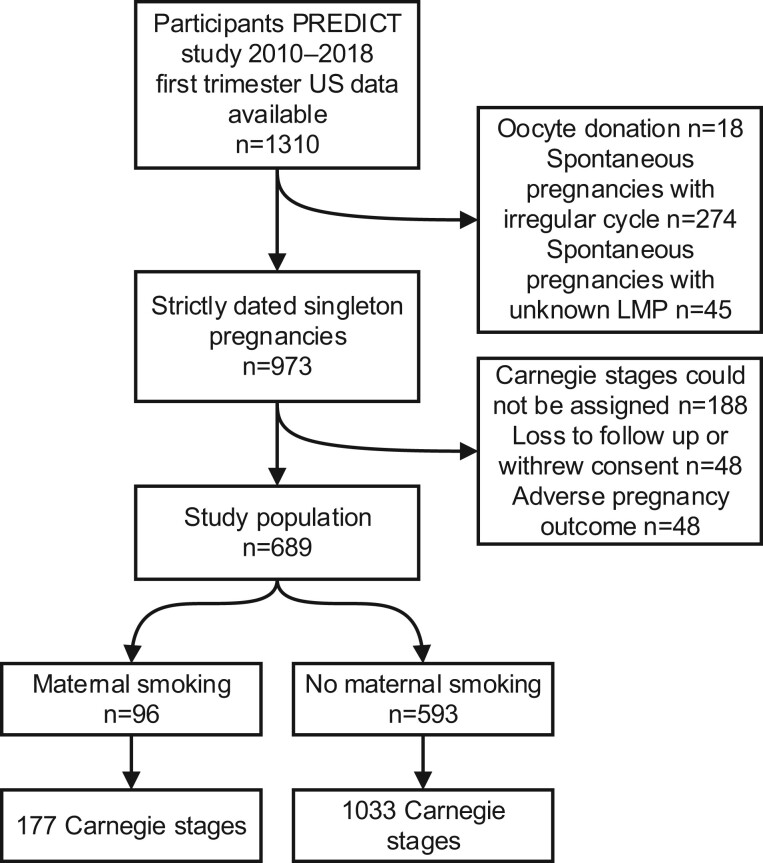
In Fig. 2, the flowchart of the study population is depicted. From a total of 1310 pregnancies with available 3D ultrasound volumes, 621 pregnancies were excluded. In total 689 pregnancies, of 96 smoking women and 593 non-smoking women were included, with 1210 assigned Carnegie stages.
Figure 2.
Flow chart of the study population.
Baseline characteristics are shown in Table I. The three groups were significantly different for maternal age (P = 0.002), maternal BMI (P = 0.032), educational level (P < 0.001), alcohol usage during pregnancy (P < 0.001) and periconceptional folic acid supplementation (P = 0.003). Of the smoking women, 41% conceived through IVF/ICSI, whereas 51% of the non-smoking group conceived through IVF/ICSI (P = 0.070). Birth weight percentile was significantly different for the groups (P = 0.004). Smoking women more often (19% for 1–9 cigarettes/day and 36% for ≥10 cigarettes/day) had SGA children compared to non-smoking women (12%) (P < 0.001).
Table I.
Baseline characteristics of participants.
| Maternal characteristics | Smoking women |
Non-smoking women |
P | ||||
|---|---|---|---|---|---|---|---|
| N = 96 |
N = 593 |
||||||
| Smoking 1–9 cigarettes/day N = 60 | Missing | Smoking ≥10 cigarettes/day N = 36 | Missing | Missing | |||
| Age, years, mean (±SD) | 31.1 (4.4) | 0 | 30.7 (4.3) | 0 | 32.6 (4.4) | 0 | 0.002* |
| Nulliparous, total N (%) | 37 (62) | 0 | 21 (58) | 0 | 321 (54) | 0 | 0.493 |
| BMI, kg/m2, median (IQR) | 24.2 (21.8–27.6) | 0 | 26.9 (23.3–30.0) | 0 | 24.0 (21.8–27.6) | 0 | 0.032* |
| Geographic origin | 0 | 0 | 0 | 0.558 | |||
| Western, N (%) | 51 (85) | 33 (92) | 505 (85) | ||||
| Non-western, N (%) | 9 (15) | 3 (8) | 88 (15) | ||||
| Educational level | 2 | 0 | 9 | <0.001* | |||
| Low, N (%) | 3 (5) | 6 (17) | 42 (7) | ||||
| Middle, N (%) | 32 (55) | 18 (50) | 185 (32) | ||||
| High, N (%) | 23 (40) | 12 (33) | 357 (61) | ||||
| Alcohol, total, N (%) | 36 (60) | 0 | 16 (44) | 0 | 155 (26) | 0 | <0.001* |
| Periconceptional folic acid, total, N (%) | 41 (68) | 0 | 29 (81) | 0 | 516 (87) | 0 | 0.003* |
| Vitamin use, N (%) | 38 (64) | 1 | 22 (61) | 0 | 397 (67) | 0 | 0.705 |
| Mode of conception | 0 | 0 | 0 | 0.070 | |||
| Naturally conceived, N (%) | 39 (65) | 18 (50) | 293 (49) | ||||
| IVF/ICSI conceived, N (%) | 21 (35) | 18 (50) | 300 (51) | ||||
| 1st trimester ultrasound | |||||||
| Available measurements per participant, median (IQR) | 2 (2–3) | 0 | 2 (1–3) | 0 | 2 (1–3) | 0 | 0.204 |
| 2nd trimester ultrasound | |||||||
| Head circumference Z-score, mean (±SD) | 0.48 (0.86) | 3 | 0.11 (0.86) | 2 | 0.17 (0.81) | 49 | 0.026* |
| Abdominal circumference Z-score, mean (±SD) | 0.82 (0.96) | 3 | 0.60 (0.91) | 2 | 0.80 (0.87) | 50 | 0.425 |
| Femur length Z-score, mean (±SD) | 0.30 (0.91) | 3 | 0.06 (1.02) | 2 | 0.39 (0.88) | 50 | 0.106 |
| Birth outcomes | |||||||
| Birth weight, g, median (IQR) | 3240 (2875–3485) | 1 | 3330 (2685–3580) | 1 | 3375 (3045–3706) | 15 | 0.062 |
| Birth weight, percentiles, median (IQR) | 36.5 (14.3–63.5) | 2 | 27 (5.5–59.5) | 1 | 47 (24–75) | 15 | 0.004* |
| Gestational age at birth, median (IQR) | 271 (265–280) | 2 | 274 (265–284) | 1 | 274 (267–282) | 15 | 0.834 |
| Small for gestational age (<10th percentile), N (%) | 11 (19) | 2 | 12 (36) | 1 | 65 (12) | 45 | <0.001* |
| Males, N (%) | 29 (48) | 0 | 24 (67) | 0 | 283 (49) | 9 | 0.104 |
Significance at P ≤ 0.05 assessed by ANOVA.
IQR, interquartile range.
Smoking and embryonic morphological development
In Table II, the association between smoking and Carnegie stages is depicted. The number of cigarettes/day was associated with a reduced expected value of embryonic morphological development, reflected by a delay in Carnegie stages seen in Model 1 (βcigarettes/day = −0.067, 95% CI −0.129; −0.006, P = 0.031). In Model 2, after adjustment for confounders, the association was no longer significant (βcigarettes/day = −0.058, 95% CI −0.122; 0.007, P = 0.080). Smoking of ≥10 cigarettes/day, compared to non-smoking women, was associated with a lower expected value of the Carnegie stages in both models (Model 1, β≥10 cigarettes/day = −0.398, 95% CI −0.686; −0.111, P = 0.007; Model 2, β≥10 cigarettes/day = −0.352, 95% CI −0.648; −0.057, P = 0.019).
Table II.
Associations between maternal periconceptional smoking and Carnegie stages.
| Model 1a | P | Model 2b | P | |
|---|---|---|---|---|
| Effect estimate (β), 95% CI | Effect estimate (β), 95% CI | |||
| Smoking | ||||
| No | 0 (Reference) | 0 (Reference) | ||
| Yes | −0.094 (−0.278; 0.089) | 0.310 | −0.052 (−0.247; 0.143) | 0.601 |
| Cigarettes/day | −0.067 (−0.129; −0.006) | 0.031* | −0.058 (−0.122; 0.007) | 0.080 |
| Smoking, categorical | ||||
| None | 0 (Reference) | 0 (Reference) | ||
| 1–9 cigarettes/day | 0.082 (−0.142; 0.306) | 0.472 | 0.129 (−0.107; 0.365) | 0.284 |
| ≥10 cigarettes/day | −0.398 (−0.686; −0.111) | 0.007* | −0.352 (−0.648; −0.057) | 0.019* |
P < 0.05 (bold text).
Model with GA as time predictor.
Fully adjusted model with GA as time predictor; adjusted for alcohol use, educational level, folic acid supplement use, maternal age, mode of conception, ethnicity, fetal sex, maternal BMI, parity and vitamin use.
Analysis stratified for mode of conception and fetal sex
Table III shows the association between smoking and Carnegie stages for the naturally conceived group and IVF/ICSI group (n = 339 pregnancies) separately. Smoking (yes) was associated with a delayed embryonic morphological development in Model 1 (βsmoking (yes) = −0.263, 95% CI −0.479; −0.047, P = 0.017) and in Model 2 (βsmoking (yes) = −0.305, 95% CI −0.544; −0.067, P = 0.012). The number of cigarettes/day was associated with a lower expected value of the Carnegie stages in Model 1 (βcigarettes/day = −0.112, 95% CI −0.181; −0.043, P = 0.001) and Model 2 (βcigarettes/day = −0.126, 95% CI −0.200; −0.051, P = 0.001), with a dose–response effect. Periconceptional smoking of ≥10 cigarettes/day was associated with a lower expected value of embryonic morphological development (Model 2, β≥10 cigarettes/day = −0.510, 95% CI −0.834; −0.186, P = 0.002).
Table III.
Stratified analysis for mode of conception: associations between maternal periconceptional smoking and Carnegie stages for IVF/ICSI conceived pregnancies.
| Model 1a | P | Model 2b | P | ||
|---|---|---|---|---|---|
| Effect estimate (β), 95% CI | Effect estimate (β), 95% CI | ||||
| Naturally conceived (N = 350) | Smoking | ||||
| No (N = 293) | 0 (Reference) | 0 (Reference) | |||
| Yes (N = 53) | 0.068 (−0.213; 0.349) | 0.635 | 0.164 (−0.132; 0.459) | 0.276 | |
| Cigarettes/day | −0.020 (−0.118; 0.077) | 0.683 | 0.009 (−0.093; 0.111) | 0.867 | |
| Smoking, categorical | |||||
| None (N = 293) | 0 (Reference) | 0 (Reference) | |||
| 1–9 cigarettes/day (N = 39) | 0.232 (−0.096; 0.561) | 0.166 | 0.313 (−0.030; 0.656) | 0.073 | |
| ≥10 cigarettes/day (N = 18) | −0.298 (−0.773; 0.176) | 0.217 | −0.170 (−0.659; 0.320) | 0.496 | |
| IVF/ICSI conceived (N = 339) | Smoking | ||||
| No (N = 300) | 0 (Reference) | 0 (Reference) | |||
| Yes (N = 39) | −0.263 (−0.479; −0.047) | 0.017* | −0.305 (−0.544; −0.067) | 0.012* | |
| Cigarettes/day | −0.112 (−0.181; −0.043) | 0.001* | −0.126 (−0.200; −0.051) | 0.001* | |
| Smoking, categorical | |||||
| None (N = 300) | 0 (Reference) | 0 (Reference) | |||
| 1–9 cigarettes/day (N = 21) | −0.082 (−0.366; 0.203) | 0.572 | −0.131 (−0.443; 0.181) | 0.409 | |
| ≥10 cigarettes/day (N = 18) | −0.479 (−0.787; −0.170) | 0.002* | −0.510 (−0.834; −0.186) | 0.002* |
P < 0.05 (bold text).
Model with gestational age as time predictor.
Fully adjusted model with gestational age as time predictor; adjusted for alcohol use, educational level, folic acid supplement use, maternal age, ethnicity, fetal sex, maternal BMI, parity and vitamin use.
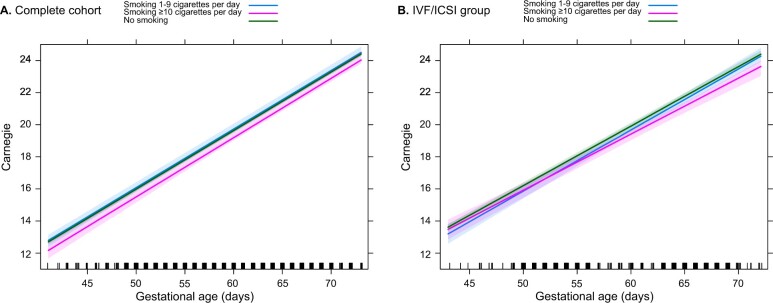
In Fig. 3a, the delay in Carnegie stages is displayed comparing non-smoking women and those who smoked ≥10 cigarettes/day. In the complete cohort, an embryo of a woman smoking ≥10 cigarettes/day will reach the final Carnegie stage with a delay of 0.9 day compared to non-smoking women. Figure 3b shows the IVF/ICSI group; smoking of ≥10 cigarettes/day resulted in a 1.6-day delay in embryonic morphological development.
Figure 3.
Carnegie stages in non-smoking and smoking pregnancies. Complete cohort (A) and IVF/ICSI group (B), with color coding reflecting non-smoking women and smoking women according to the number of cigarettes/per day. The green, blue and pink lines respectively represent the non-smokers, periconceptional smoking of 1–9 cigarettes/day and periconceptional smoking of ≥10 cigarettes/day. In the complete cohort, an embryo of a woman smoking ≥10 cigarettes/day will reach the final Carnegie stage with a delay of 0.9 day compared to non-smoking women. In the IVF/ICSI group, smoking of ≥10 cigarettes/day resulted in a 1.6 day delay in embryonic morphological development.
Supplementary Table SI shows the association between smoking and Carnegie stages, stratified for fetal sex. A trend toward delayed morphological development was found. Interaction between embryonic morphology, maternal smoking and fetal sex was absent: Pinteraction = 0.329 for smoking as a dichotomous (yes/no) variable, Pinteraction = 0.204 for smoking as a continuous variable (number cigarettes/day) and Pinteraction = 0.447 for smoking as a categorized variable (control versus 1–9 cigarettes/day versus ≥10 cigarettes/day).
Smoking and fetal growth
The association between periconceptional smoking and second-trimester fetal growth is shown in Table IV. Periconceptional smoking (yes/no) was not significantly associated with second-trimester fetal growth. Maternal smoking represented by number of cigarettes/day was associated with a smaller femur length after adjustment in Model 2 (βcigarettes/day = −0.077, 95% CI −0.147; −0.008, P = 0.029). The association between femur length was most apparent in the category ‘smoking ≥10 cigarettes/day’ (Model 2, β≥10 cigarettes/day = −0.321, 95% CI −0.641; −0.001, P = 0.049). Smoking of 1–9 cigarettes/day was associated with a larger head circumference in the second trimester (Model 2, β1–9 cigarettes/day = 0.290, 95% CI 0.065; 0.514, P = 0.012).
Table IV.
Associations between smoking (continuous and categorical variable) and fetal growth parameters: second trimester head circumference, abdominal circumference femur length and birth weight.
| Model 1a | P | Model 2b | P | |
|---|---|---|---|---|
| Effect estimate (β), 95% CI | Effect estimate (β), 95% CI | |||
| Head circumference | ||||
| Cigarettes/day | 0.010 (−0.051; 0.071) | 0.743 | −0.003 (−0.064; 0.058) | 0.924 |
| Smoking None | 0 (Reference) | 0 (Reference) | ||
| 1–9 cigarettes/day | 0.283 (0.056; 0.510) | 0.015* | 0.290 (0.065; 0.514) | 0.012* |
| ≥10 cigarettes/day | −0.069 (−0.353; 0.214) | 0.631 | −0.134 (−0.413; 0.144) | 0.345 |
| Abdominal circumference | ||||
| Cigarettes/day | −0.043 (−0.110; 0.031) | 0.279 | −0.053 (−0.121; 0.015) | 0.129 |
| Smoking None | 0 (Reference) | 0 (Reference) | ||
| 1–9 cigarettes/day | 0.000 (−0.250; 0.249) | 0.997 | 0.023 (−0.231; 0.277) | 0.861 |
| ≥10 cigarettes/day | −0.199 (−0.511; 0.113) | 0.211 | −0.261 (−0.576; 0.054) | 0.104 |
| Femur length | ||||
| Cigarettes/day | −0.077 (−0.144; −0.011) | 0.022* | −0.077 (−0.147; −0.008) | 0.029* |
| Smoking None | 0 (Reference) | 0 (Reference) | ||
| 1–9 cigarettes/day | −0.111 (−0.360; 0.137) | 0.380 | −0.095 (−0.353; 0.163) | 0.471 |
| ≥10 cigarettes/day | −0.323 (−0.633; −0.012) | 0.042* | −0.321 (−0.641; −0.001) | 0.049* |
| Birth weight | ||||
| Cigarettes/day | −0.145 (−0.227; −0.063) | 0.001* | −0.150 (−0.233; −0.068) | 0.000* |
| None | 0 (Reference) | 0 (Reference) | ||
| 1–9 cigarettes/day | −0.314 (−0.621; −0.008) | 0.044* | −0.306 (−0.612; 0.001) | 0.050 |
| ≥10 cigarettes/day | −0.519 (−0.904; −0.135) | 0.008* | −0.560 (−0.941; −0.178) | 0.004* |
Growth parameters are expressed as Z-scores, which are corrected for gestational age. Birth weight is expressed as Z-score, which is corrected for gender and gestational age.
P < 0.05 (bold text).
Crude model.
Fully adjusted model adjusted for alcohol use, educational level, folic acid supplement use, maternal age, mode of conception, ethnicity, fetal gender, maternal BMI, parity and vitamin use.
Table IV also shows the association between periconceptional smoking and birth weight. The number of cigarettes/day was associated with lower birth weight in Model 2 (βcigarettes/day = −0.150, 95% CI −0.233; −0.068, P < 0.001). Compared to non-smoking women, periconceptional smoking of ≥10 cigarettes/day was associated with the highest birth weight reduction (Model 2, β≥10 cigarettes/day = −0.560, 95% CI −0.941; −0.178, P = 0.004). At 40 weeks GA, maternal smoking of ≥10 cigarettes/day resulted in an estimated reduction in birth weight of 228 g in a male infant and an estimated reduction of 218 g in a female infant.
Effect of embryonic morphology on fetal growth
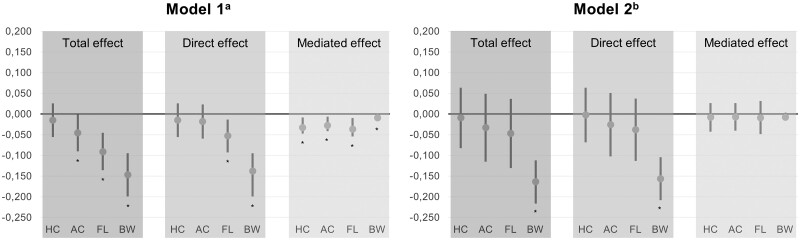
The estimated mediated effect of embryonic morphology on second-trimester fetal growth parameters and birth weight are depicted in Fig. 4 for Models 1 and 2 separately. A significant mediation effect of delayed embryonic morphology on the association between smoking and all fetal growth parameters was seen in Model 1. The proportion explained by embryonic morphology varied from 41.3% (95% CI 19.4; 73.6) for femur length and up to 61.1% (95% CI 22.4; 295.0) for abdominal circumference, as seen in Supplementary Table SII, Model 1. The mediated proportion for head circumference in Model 1 could not be interpreted due to opposite direct and indirect effect estimates. No significant total or mediation effect was noted on fetal growth parameters in Model 2, available in Supplementary Table SII. The estimated mediated effect of delayed embryonic morphology due to the association between smoking and birth weight was significant in Model 1 (βcigarettes/day = −0.009, 95% CI −0.014; −0.002), but not in Model 2 (βcigarettes/day = −0.008, 95% CI −0.012; 0.001). The mediated proportion was 6.3% in Model 1 (95% CI 2.0; 14.5) and 4.8% in Model 2 (95% CI −0.8; 7.6).
Figure 4.
Mediation analysis showing that part of the effect of periconceptional smoking on fetal growth parameters and birth weight can be explained by a delayed embryonic morphology. The asterisk (*) below the bar indicates significant effect. Note that the mediated proportion for head circumference in Model 1 could not actually be interpreted due to opposite direct and indirect effect estimates. 1a Crude model. 2b Model adjusted for alcohol use, educational level, folic acid supplement use, maternal age, mode of conception, ethnicity, fetal sex, maternal BMI, parity and vitamin use. AC, abdominal circumference; BW, birth weight; FL, femur length; HC, head circumference. Growth parameters are expressed as Z-scores, which are corrected for gestational age. Birth weight is expressed as Z-score, which is corrected for sex and gestational age. The total effect of smoking on fetal growth parameters and birth weight is reported by effect estimates (β) with 95% CIs and is decomposed into a direct effect and an indirect effect mediated by Carnegie developmental stages.
Discussion
Maternal smoking in the periconceptional period is associated with a delay in embryonic morphological development when assessed in utero by the Carnegie staging system. We found a 0.9-day delay in morphological development in the total group and a 1.6-day delay in the IVF/ICSI group as a result of periconceptional maternal smoking (≥10 cigarettes/day). Furthermore, smoking in the periconceptional period is associated with smaller fetal growth reflected by second-trimester femur length and lower birth weight. In the unadjusted model, up to 61.1% of the association between smoking and fetal growth and 6.3% of the association between smoking and birth weight can be explained by the delay in Carnegie stages.
First-trimester findings
The findings of the current study are in line with a previous study in a smaller subset of the same cohort focusing on the effects of smoking on embryonic growth in the first trimester (van Uitert et al., 2013). The CRL of embryos of women who smoked ≥10 cigarettes/day in the periconceptional period were 18.7% and 5.5% smaller at 6 and 12 weeks GA, respectively compared to embryos of non-smokers (van Uitert et al., 2013). Notwithstanding differences between size reflected by CRL and Carnegie stages, smoking in the periconceptional period has a similar negative effect on both embryonic growth and morphology. In a study by Mongelli et al. (2016), CRL was smaller in women who smoked during pregnancy with repeated measurements between 5 and 14 weeks GA. Their population was retrospectively selected, with 55% of women presenting with first-trimester bleeding. In contrast, another study including single CRL measurements of 900 pregnant women between 8 and 12 weeks GA, showed no association between maternal smoking and CRL (Prabhu et al., 2010). This study however did not include repeated first-trimester measurements, and therefore an association between smoking and CRL might have been lost. Interestingly, they did find a reduction in femur length in the second trimester, as seen in the current study.
Other human studies focusing on morphology are limited, as we found only the study by Lutterodt et al. (2009). Our findings regarding embryonic morphological development are in contrast with their study results. They studied the association between smoking and the development of feet and foot length in 72 aborted embryos and found no association. However, only the feet were studied in relation to tobacco exposure, whereas we evaluated morphology of the complete embryo using the Carnegie staging system. In other interesting studies, new parameters for embryonic development are introduced like in the study by Fowler et al. (2016) where they show that maternal smoking is associated with longer anogenital distance in selectively terminated embryos and fetuses. These studies provide us with additional insights into human embryonic development.
In the current study, we showed a 1.6-day delay in reaching the final Carnegie stage of the IVF/ICSI pregnancies of women who smoked ≥10 cigarettes/day. The compelling effect in the IVF/ICSI population may be due to very strict dating of the pregnancy. On the other hand, the effect of tobacco smoke might also be amplified in this group. Maternal smoking decreases fertilization, pregnancy and live birth rates in the IVF population (Klonoff-Cohen, 2005). Moreover, it is thought that smoking might cause embryonic and fetal growth restriction via reduced early placental development (Practice Committee of the American Society for Reproductive Medicine, 2018). On the other hand, it is also said that due to the technique itself, IVF/ICSI pregnancies are more susceptible to external exposures and thus have an increased risk for aberrant growth, causing SGA and lower birth weight (van Uitert et al., 2013; Berntsen et al., 2019). Moreover, smoking pregnant women who conceived through IVF/ICSI are exposed to a combination of both the risks related to the ART procedure and the compounds of tobacco smoke causing further impaired development.
Second-trimester and birth weight findings
We found a smaller fetal femur length in the group of smoking women but did not find any associations with abdominal circumference and head circumference. In a meta-analysis by Abraham et al. (2017), smoking during pregnancy resulted in similar smaller second-trimester fetal measurements of abdominal circumference and femur length. In their analysis of over 11 000 second-trimester ultrasound measurements, the head circumference was also smaller in the second trimester. Although this association might be apparent in a large meta-analysis, we were unable to detect a similar association in our smaller cohort. Additionally, when we analyzed fetal head circumference and smoking per category, we found a larger head circumference in the group of 1–9 cigarettes/day, compared to non-smoking women. However, smoking more cigarettes, ≥10 cigarettes/day was associated with a smaller head circumference compared to non-smoking women. The inversion of the association can be explained by a small sample size. Earlier findings in the literature describe a negative association between smoking and fetal head circumference explained by a biological mechanism (Andersen et al., 2009). A dose–response relationship was not investigated. Recently, folic acid supplementation was found to reduce the risk of having impaired brain–body proportionality in smoking pregnant women (Yusuf et al., 2019). The role of folic acid cannot be ruled out as almost all women used folic acid supplementation; however, in our mixed linear model, we did adjust for oral folic acid supplementation.
Using mediation analysis with the unadjusted model, we found that 41.3% of the association between smoking and femur length in the second trimester and 61.1% of the association between smoking and abdominal circumference in the second trimester can be explained by a delay in embryonic morphology. In the fully adjusted model, no significant associations were found. The significant association of Z-transformed values of the crude analysis were rather small, and thus might have been lost after adjustment for known covariates and confounders. Still, 40–60% of the association between smoking and fetal growth parameters can be explained by the delay in the embryonic morphology, indicating that the detrimental effects of smoking on fetal growth can only partially be recuperated. In addition, the mediation analysis shows the proportions vary for the different measurements of fetal body parts. These different proportional effects may suggest that smoking may have a different effect on various body parts of the fetus. However, future studies should further clarify these associations found.
In our study, we found a lower median birth weight of 93 g, a higher percentage of SGA, and a lower GA-adjusted birth weight in pregnancies of smoking women. Our effect on median birth weight is larger than reported in a meta-analysis in which, however, only non-adjusted mean birth weight was included (Leonardi-Bee et al., 2008). Our results regarding GA-adjusted birth weight are in line with other studies on periconceptional smoking status (Cardenas et al., 2019). In a recent meta-analysis, the authors noted that information on number of cigarettes per day was scarcely reported in the included studies and it was impossible to stratify by number of cigarettes per day or categories (Pereira et al., 2017). For birth weight in Model 1, we noticed a significant effect of ∼6% of the association of smoking can be explained by the delay in embryonic morphology. This may imply a small effect of the delayed morphology already caused by periconceptional smoking in the first trimester, which is not recuperated later in pregnancy.
Strengths and limitations
The main strength of this study is the large number of pregnancies with a certain pregnancy dating and the availability of serial first-trimester ultrasound data. Adequate pregnancy dating is essential to reduce confounding by GA. Furthermore, unique for this study is the knowledge on periconceptional smoking as exposure. This offers us the opportunity to relate both lifestyle and environmental factors as exposures to embryonic and fetal development. Finally, we used 3D VR to assign Carnegie stages in utero. The 3D VR approach provides extra information, on top of routine clinical 2D ultrasound, and offers a high degree of precision and reliability for volumetric measurements and embryonic morphological examination (Rousian et al., 2018).
Our study population was recruited from a tertiary referral center, including women with co-morbidities such as cardiovascular, autoimmune, endocrine and metabolic diseases. Additionally, the women were highly educated and many used folic acid supplementation. Consequently, results cannot be extrapolated to the general population. Inherent to the design of the study is the selective participation of a large group of IVF/ICSI pregnancies, which also confines external validity. The study is embedded in the outpatient clinic, and it is therefore not feasible to record participation rate and this may be a potential risk for confounding due to selection bias. Furthermore, smoking habits were explored using self-reported questionnaires. Women might be reluctant to fill out correct smoking habits and this may cause an underestimation of the true effect of maternal smoking on embryonic morphological development and fetal growth. For example in Table III for the naturally conceived pregnancies, the trend for the association of smoking 1–9 cigarettes/day on the Carnegie stages is positive. An explanation for this finding most likely is the non-smoking women group contains women who continued to smoke during the periconceptional period but did not give a true answer. Preferably, smoking would be analyzed with a urine cotinine assay (Shipton et al., 2009), which could improve the measurement of true exposure to tobacco smoke in the periconceptional period. Information on smoking cessation or reducing the number of cigarettes would be insightful, but unfortunately, this information is not available.
We were able to show a dose–response effect of maternal smoking on embryonic morphology and fetal growth, demonstrating developmental delay with an increasing number of smoked cigarettes/day. However, in the group of 1–9 cigarettes/day, the association between smoking and embryonic morphology was not significant, most likely due to the small sample size. The total number of smokers is relatively small and therefore our results have a limited statistical power.
We were limited to studying human morphology using Carnegie stages in the first 60 days after conception. Currently, no description of morphology exists from completion of the embryonic period onwards. As a proxy for fetal morphology in our study, we used fetal growth reflected by ultrasound parameters in the second trimester and weight at birth, although these markers do not exactly describe morphological development.
Implication for practice
This is the first study to examine the influence of smoking on embryonic morphology. Tobacco smoke contains many substances that affect fertility, pregnancy and live birth rates (Dechanet et al., 2011). We show that, besides growth, embryonic morphological development is also delayed by periconceptional tobacco smoking. The exact mechanism of action on embryonic development remains unknown but a multifactorial process is likely to explain the biological effect of smoking on embryonic morphological development.
Hence, early pregnancy is a time window crucial for optimal embryonic development and even long-term health outcomes. Smoke-free legislation has already shown to reduce the incidence of preterm birth (Faber et al., 2017). A next step to improve pregnancy outcome and optimize periconceptional risk factors by using the effective mHealth lifestyle coaching program www.smarterpregnancy.co.uk. This online program has shown to improve inadequate lifestyle behavior (Oostingh et al., 2020). This study emphasizes the importance of the periconceptional period and preconception information on smoking cessation for women trying to conceive.
The Carnegie stages may be used to inform future parents about early morphological development. Furthermore, morphological development might be used as a more reliable measure for health and development in the future.
Conclusion
This study shows that the impact of periconceptional maternal smoking on human development can already be detected early in the first trimester of pregnancy using embryonic morphology as outcome. The Carnegie staging system takes the whole embryo into account and might be a more reliable measure than 2D embryonic size for health and development in the future. One of the key messages of this study is that delay, or dysregulation, in embryonic morphology is associated with allometric growth reflected by smaller fetal measurements at 20 weeks gestation and lower weight at birth. The delay in embryonic morphology, measured in early pregnancy, cannot be recuperated during the pregnancy. The results of this study emphasize the importance of smoking intervention programs prior to conception. More research is warranted to assess the association between periconceptional smoking cessation and embryonic development.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
Acknowledgements
We would thank all of the participating women and the whole Rotterdam Periconception Cohort group for the great efforts from 2009 onwards.
Authors’ roles
C.S.P. contributed to data collection, analysis and interpretation, and wrote the first draft and revised all versions of the manuscript. M.R. and A.S. performed embryonic morphological measurements. S.P.W. analyzed data and contributed to the interpretation of results. M.S.J. contributed to data collection. A.G.M.G.J.M., R.P.M.S-T. and E.A.P.S. supervised the interpretation of the results and writing of the manuscript. R.P.M.S-T. is principal investigator of the Rotterdam Periconception cohort. M.R. had primary responsibility for final content and supervised and contributed to all aspects of this study. All authors read and approved the final manuscript.
Funding
The work was funded by the Department of Obstetrics and Gynecology, Erasmus MC, University Medical Center, Rotterdam, the Netherlands.
Conflict of interest
No conflict of interest has to be declared by any of the authors regarding the material discussed in the manuscript.
References
- Abraham M, Alramadhan S, Iniguez C, Duijts L, Jaddoe VW, Den Dekker HT, Crozier S, Godfrey KM, Hindmarsh P, Vik T. et al. A systematic review of maternal smoking during pregnancy and fetal measurements with meta-analysis. PLoS One 2017;12:e0170946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen MR, Simonsen U, Uldbjerg N, Aalkjær C, Stender S.. Smoking cessation early in pregnancy and birth weight, length, head circumference, and endothelial nitric oxide synthase activity in umbilical and chorionic vessels: an observational study of healthy singleton pregnancies. Circulation 2009;119:857–864. [DOI] [PubMed] [Google Scholar]
- Berntsen S, Soderstrom-Anttila V, Wennerholm UB, Laivuori H, Loft A, Oldereid NB, Romundstad LB, Bergh C, Pinborg A.. The health of children conceived by ART: ‘the chicken or the egg?’. Hum Reprod Update 2019;25:137–158. [DOI] [PubMed] [Google Scholar]
- Blaas HG, Eik-Nes SH, Berg S, Torp H.. In-vivo three-dimensional ultrasound reconstructions of embryos and early fetuses. Lancet 1998;352:1182–1186. [DOI] [PubMed] [Google Scholar]
- Cardenas A, Lutz SM, Everson TM, Perron P, Bouchard L, Hivert MF.. Mediation by placental DNA methylation of the association of prenatal maternal smoking and birth weight. Am J Epidemiol 2019;188:1878–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centraal Bureau voor Statistiek. The Dutch Standard Classification of Education 2016. 2016. https://www.cbs.nl/nl-nl/onze-diensten/methoden/classificaties/onderwijs-en-beroepen/standaard-onderwijsindeling-soi-/standaard-onderwijsindeling-2016 (30 May 2021, date last accessed).
- Dechanet C, Anahory T, Mathieu Daude JC, Quantin X, Reyftmann L, Hamamah S, Hedon B, Dechaud H.. Effects of cigarette smoking on reproduction. Hum Reprod Update 2011;17:76–95. [DOI] [PubMed] [Google Scholar]
- Dutch Society for Obstetrics and Gynecology. Guideline for Structural Ultrasound Examination Version 3.0. 2019. https://www.nvog.nl/wp-content/uploads/2019/07/Structureel-Echoscopisch-Onderzoek-SEO-23-07-2019.pdf (8 June 2020, date last accessed).
- Faber T, Kumar A, Mackenbach JP, Millett C, Basu S, Sheikh A, Been JV.. Effect of tobacco control policies on perinatal and child health: a systematic review and meta-analysis. Lancet Public Health 2017;2:e420–e437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farland LV, Correia KFB, Dodge LE, Modest AM, Williams PL, Smith LH, Toth TL, Hacker MR, Missmer SA.. The importance of mediation in reproductive health studies. Hum Reprod 2020;35:1262–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler PA, Filis P, Bhattacharya S, Le Bizec B, Antignac JP, Morvan ML, Drake AJ, Soffientini U, O'Shaughnessy PJ.. Human anogenital distance: an update on fetal smoke-exposure and integration of the perinatal literature on sex differences. Hum Reprod 2016;31:463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackshaw A, Rodeck C, Boniface S.. Maternal smoking in pregnancy and birth defects: a systematic review based on 173 687 malformed cases and 11.7 million controls. Hum Reprod Update 2011;17:589–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoftiezer L, Hof MHP, Dijs-Elsinga J, Hogeveen M, Hukkelhoven C, van Lingen RA.. From population reference to national standard: new and improved birthweight charts. Am J Obstet Gynecol 2019;220:383.e1–383.e17. [DOI] [PubMed] [Google Scholar]
- Kataria Y, Gaewsky L, Ellervik C.. Prenatal smoking exposure and cardio-metabolic risk factors in adulthood: a general population study and a meta-analysis. Int J Obes (Lond) 2019;43:763–773. [DOI] [PubMed] [Google Scholar]
- Klonoff-Cohen H. Female and male lifestyle habits and IVF: what is known and unknown. Hum Reprod Update 2005;11:179–203. [DOI] [PubMed] [Google Scholar]
- Lange S, Probst C, Rehm J, Popova S.. National, regional, and global prevalence of smoking during pregnancy in the general population: a systematic review and meta-analysis. Lancet Glob Health 2018;6:e769–e776. [DOI] [PubMed] [Google Scholar]
- Leonardi-Bee J, Smyth A, Britton J, Coleman T.. Environmental tobacco smoke and fetal health: systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 2008;93:F351–F361. [DOI] [PubMed] [Google Scholar]
- Lutterodt MC, Rosendahl M, Yding Andersen C, Skouby SO, Byskov AG.. Age determination enhanced by embryonic foot bud and foot plate measurements in relation to Carnegie stages, and the influence of maternal cigarette smoking. Hum Reprod 2009;24:1825–1833. [DOI] [PubMed] [Google Scholar]
- Mongelli M, Lu C, Reid S, Stamatopoulos N, Sankaralingam K, Casikar I, Hardy N, Condous G.. Is there a correlation between aberrant embryonic crown-rump length growth velocities and subsequent birth weights? J Obstet Gynaecol 2016;36:726–730. [DOI] [PubMed] [Google Scholar]
- O'Rahilly R, Muller F.. Developmental stages in human embryos: revised and new measurements. Cells Tissues Organs 2010;192:73–84. [DOI] [PubMed] [Google Scholar]
- Oostingh EC, Koster MPH, van Dijk MR, Willemsen SP, Broekmans FJM, Hoek A, Goddijn M, Klijn NF, van Santbrink EJP, Steegers EAP. et al. First effective mHealth nutrition and lifestyle coaching program for subfertile couples undergoing in vitro fertilization treatment: a single-blinded multicenter randomized controlled trial. Fertil Steril 2020;114:945–954. [DOI] [PubMed] [Google Scholar]
- Papageorghiou AT, Ohuma EO, Altman DG, Todros T, Cheikh Ismail L, Lambert A, Jaffer YA, Bertino E, Gravett MG, Purwar M. et al. ; International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st). International standards for fetal growth based on serial ultrasound measurements: the Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project. Lancet 2014;384:869–879. [DOI] [PubMed] [Google Scholar]
- Pereira PP, Da Mata FA, Figueiredo AC, de Andrade KR, Pereira MG.. Maternal active smoking during pregnancy and low birth weight in the Americas: a systematic review and meta-analysis. Nicotine Tob Res 2017;19:497–505. [DOI] [PubMed] [Google Scholar]
- Prabhu N, Smith N, Campbell D, Craig LC, Seaton A, Helms PJ, Devereux G, Turner SW.. First trimester maternal tobacco smoking habits and fetal growth. Thorax 2010;65:235–240. [DOI] [PubMed] [Google Scholar]
- Practice Committee of the American Society for Reproductive Medicine. Smoking and infertility: a committee opinion. Fertil Steril 2018;110:611–618. [DOI] [PubMed] [Google Scholar]
- Rousian M, Hop WC, Koning AH, van der Spek PJ, Exalto N, Steegers EA.. First trimester brain ventricle fluid and embryonic volumes measured by three-dimensional ultrasound with the use of I-Space virtual reality. Hum Reprod 2013;28:1181–1189. [DOI] [PubMed] [Google Scholar]
- Rousian M, Koster MPH, Mulders A, Koning AHJ, Steegers-Theunissen RPM, Steegers EAP.. Virtual reality imaging techniques in the study of embryonic and early placental health. Placenta 2018;64(Suppl 1):S29–S35. [DOI] [PubMed] [Google Scholar]
- Shipton D, Tappin DM, Vadiveloo T, Crossley JA, Aitken DA, Chalmers J.. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study. BMJ 2009;339:b4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegers-Theunissen RP, Verheijden-Paulissen JJ, van Uitert EM, Wildhagen MF, Exalto N, Koning AH, Eggink AJ, Duvekot JJ, Laven JS, Tibboel D. et al. Cohort Profile: the Rotterdam Periconceptional Cohort (Predict Study). Int J Epidemiol 2016;45:374–381. [DOI] [PubMed] [Google Scholar]
- United Nations Department of Economic and Social Affairs Population Division. World Population Prospects 2019. 2019. https://population.un.org/wpp/Publications/Files/WPP2019_Highlights.pdf (25 November 2020, date last accessed).
- van Uitert EM, van der Elst-Otte N, Wilbers JJ, Exalto N, Willemsen SP, Eilers PHC, Koning AHJ, Steegers EAP, Steegers-Theunissen RP.. Periconception maternal characteristics and embryonic growth trajectories: the Rotterdam Predict study. Hum Reprod 2013;28:3188–3196. [DOI] [PubMed] [Google Scholar]
- VanderWeele TJ. Mediation analysis: a practitioner's guide. Annu Rev Public Health 2016;37:17–32. [DOI] [PubMed] [Google Scholar]
- Wagijo MA, Sheikh A, Duijts L, Been JV.. Reducing tobacco smoking and smoke exposure to prevent preterm birth and its complications. Paediatr Respir Rev 2017;22:3–10. [DOI] [PubMed] [Google Scholar]
- WHO. WHO Report on the Global Tobacco Epidemic, 2019. Geneva: World Health Organization, 2019.
- Yusuf KK, Salihu HM, Wilson R, Mbah A, Sappenfield W, Bruder K, Wudil UJ, Aliyu MH.. Folic acid intake, fetal brain growth, and maternal smoking in pregnancy: a randomized controlled trial. Curr Dev Nutr 2019;3:nzz025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeve D, Regelmann MO, Holzman IR, Rapaport R.. Small at birth, but how small? The definition of SGA revisited. Horm Res Paediatr 2016;86:357–360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.