Figure 1.
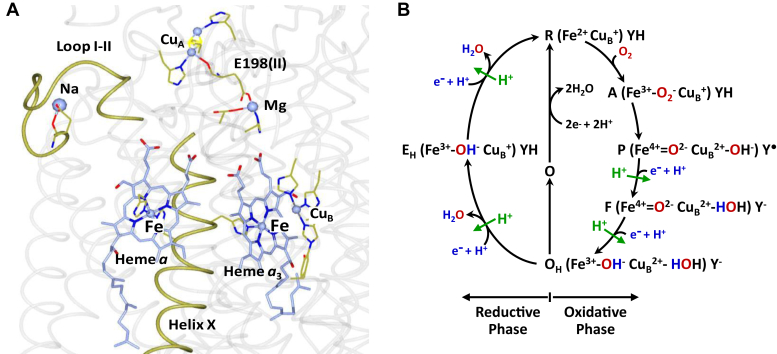
Structure of the metal centers in subunits I and II. In bCcO (A) and the proposed biphasic sequential mechanism (B). A, shows the locations of the four redox centers, CuA, heme a, CuB, and heme a3, with respect to helix X and loop I–II in bCcO. The structure is from Protein Data Bank (ID: 7TIE). The Mg atom and the E198 from subunit II that links Mg to CuA are also shown as indicated. B, shows the proposed dioxygen reduction mechanism depicting the correlation of the changes in the oxidation and ligation states of the BNC with the entry of four electrons and four substrate protons (in blue) into the BNC and the translocation of four pumped protons (in green) (see the full description in the main text). If OH produced at the end of the oxidative phase is allowed to relax to the resting O state, its reduction to R does not support proton translocation. bCcO, bovine heart CcO; BNC, binuclear center.