Figure 1. Study Route.
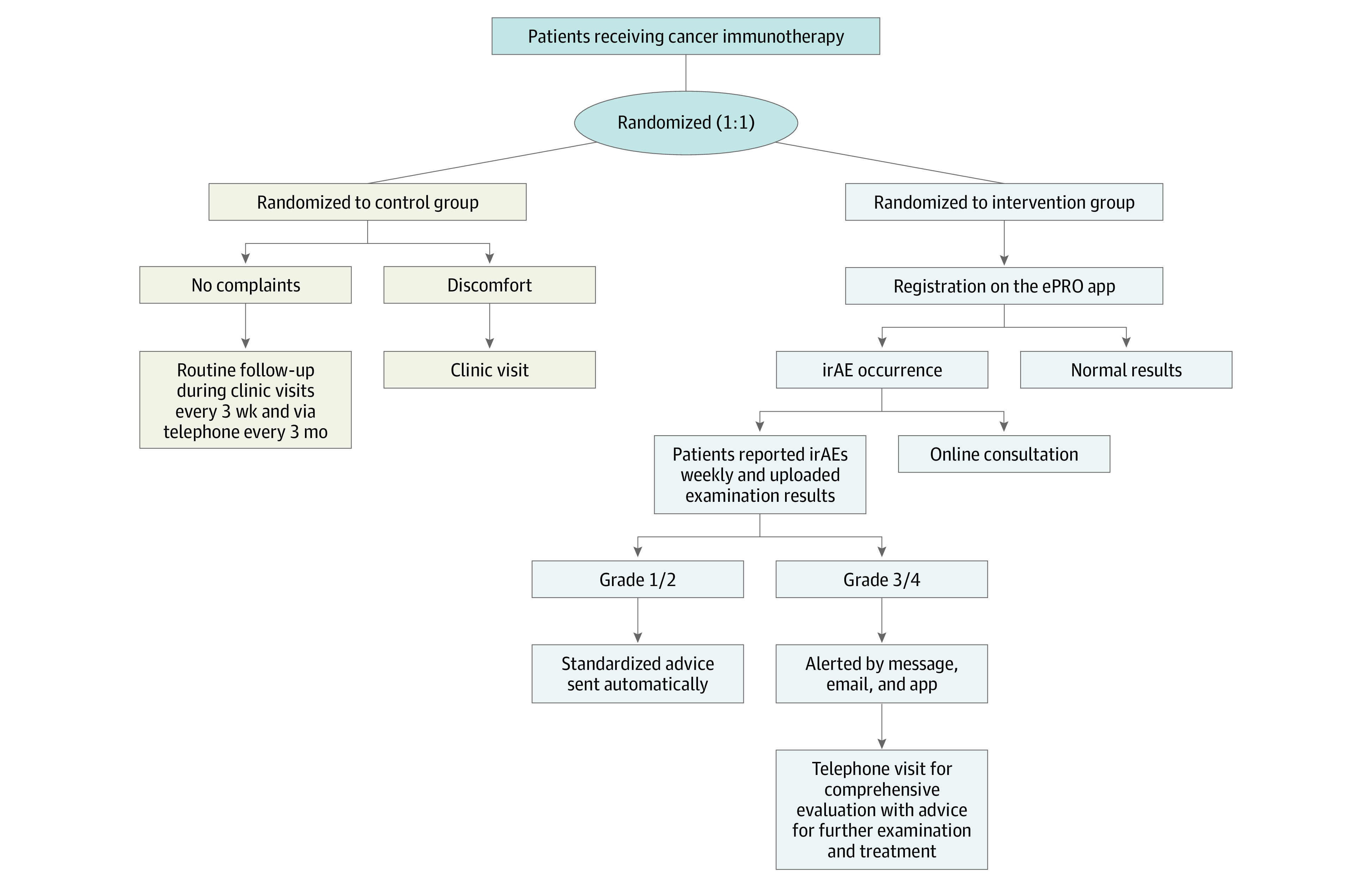
The control group received the traditional model of follow-up; the intervention group received the electronic patient-reported outcome (ePRO) model, follow-up monitoring, and consultation via the ePRO application (app). irAE indicates immune-related adverse effect; serious irAEs include grades 3 to 4.
