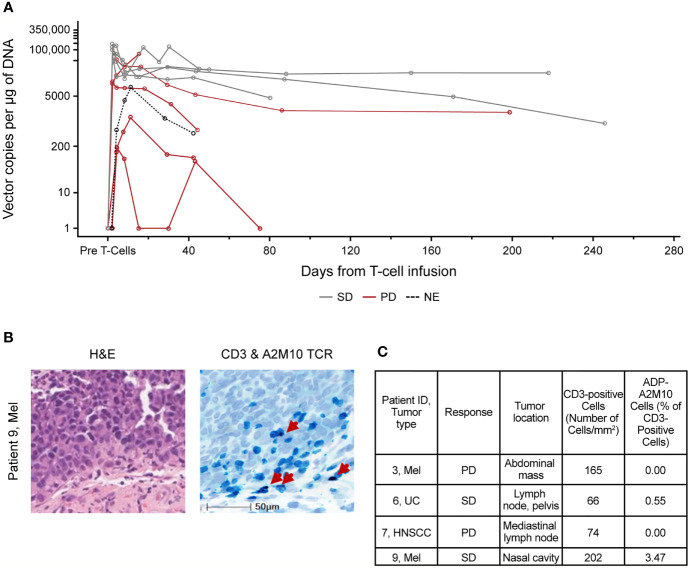
Figure 2.
ADP-A2M10 were detected in peripheral blood and tumor tissue after infusion. (A) Persistence of ADP-A2M10 was measured by quantitative PCR of the Psi element sequence in genomic DNA extracted from peripheral blood mononuclear cells. Data points are colored by response. (B) Representative fields for detection of CD3+ and/or ADP-A2M10 TCR+ cells by CD3 immunohistochemical/RNA in situ hybridization assay in tumor tissue of patient 9 collected within 12 weeks after infusion. In the right image, CD3+ cells are shown in teal, ADP-A2M10 TCR+ cells are shown in purple, and nuclei are shown in light blue (hematoxylin stain). (C) Result table for CD3 immunohistochemical/RNA in situ hybridization duplex assays reporting the detection of ADP-A2M10 in two of four post-infusion tumor samples collected from the study patients. H&E, hematoxylin and eosin stain; HNSCC, head and neck squamous cell carcinoma; Mel, melanoma; NE, not evaluable; PD, progressive disease; SD, stable disease; TCR, T-cell receptor; UC, urothelial carcinoma.