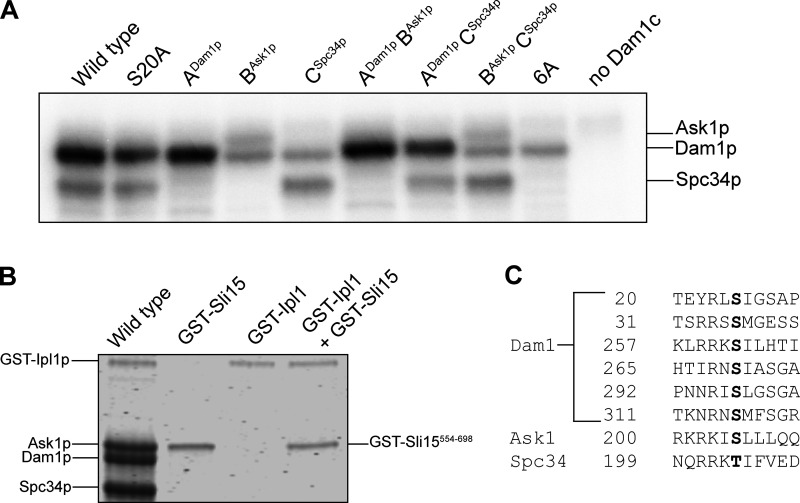
Figure S3.
Analysis of phosphorylation levels on wild-type and mutant Dam1 complexes. (A) Autoradiograph of an SDS-PAGE gel (8–14%) showing incorporation of [γ-32P]ATP into different interaction regions of the Dam1 complex. Wild-type Dam1 complex contains no alanine mutations. The S20A complex has that single mutation in Dam1p. The ADam1p complex has mutations: Dam1p S20A, Ask1p S200A, and Spc34p T199A, so only region ADam1p can be phosphorylated. The BAsk1p complex has mutations: Dam1p S20A, S257A, S265A, and S292A and Spc34p T199A, so only region BAsk1p can be phosphorylated. The CAsk1p complex has mutations: Dam1p S20A, S257A, S265A, and S292A and Ask1p S200A, so only region CSpc34p can be phosphorylated. The ADam1pBAsk1p complex has mutations: Dam1p S20A and Spc34p T199A, so only regions ADam1p and BAsk1p can be phosphorylated. The ADam1pCSpc34p complex has mutations: Dam1p S20A and Ask1p S200A, so only regions ADam1p and CAsk1p can be phosphorylated. The BAsk1pCSpc34p complex has mutations: Dam1p S20A, S257A, S265A, and S292A, so only regions BAsk1p and CSpc34p can be phosphorylated. The 6A complex has mutations: Dam1p S20A, S257A, S265A, and S292A; Ask1p S200A; and Spc34p T199A, so none of the interaction regions can be phosphorylated. The rightmost lane shows a control reaction performed in the absence of the Dam1 complex, with only Ipl1p and Sli15p. (B) Coomassie blue–stained SDS-PAGE gel (8–14%) of wild-type Dam1 complex with GST-Sli15554–698 and GST-Ipl1p (wild-type), GST-Sli15554–698 alone, GST-Ipl1p alone, and GST-Sli15554–698 together with GST-Ipl1p. (C) Highlighted in black are residues that are phosphorylated under the conditions of our phosphorylation assay. Source data are available for this figure: SourceData FS3.