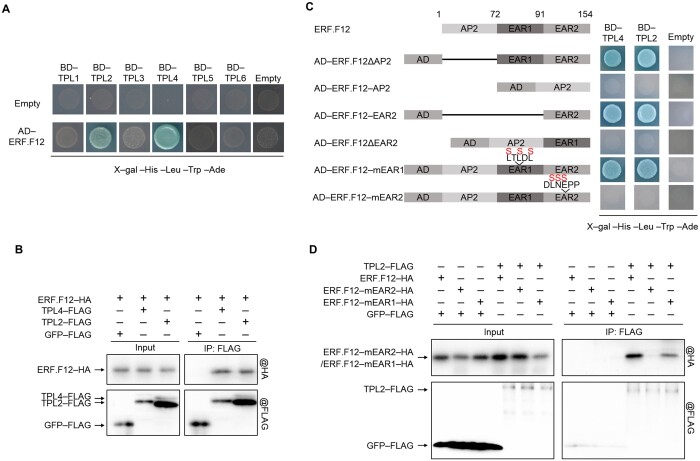
Figure 6.
ERF.F12 interacts with TPLs via the C-terminal EAR motif. A, Y2H assays between ERF.F12 and TPLs. TPL1, TPL2, TPL3, TPL4, TPL5, and TPL6 were used as bait, and ERF.F12 used as prey. Empty-BD and Empty-AD were co-transformed as negative controls. B, In vivo Co-IP assays of ERF.F12 with TPL2 and TPL4. ERF.F12-HA was transiently co-infiltrated with TPL2-FLAG or TPL4-FLAG in N. benthamiana leaves by Agrobacterium-mediated infiltration. GFP-FLAG was used as a negative control. Total proteins were extracted from infiltrated leaves and used for immunoprecipitation with anti-FLAG antibody. Immunoblots were probed with anti-FLAG antibody to detect TPLs and with anti-HA antibody to detect ERF.F12. C, The interaction between ERF.F12 and TPL2/4 is dependent on the C-terminal EAR motif. TPL2 and TPL4 were used as bait. ERF.F12 protein fragments generated by different deletions and mutated ERF.F12 in different EAR motifs were used as prey. ERF.F12ΔAP2 lacks the N-terminal AP2/ERF domain deleted; ERF.F12-AP2 harbors the AP2 domain but lacks both EAR motifs; ERF.F12-EAR2 is the C-terminal EAR motif; ERF.F12ΔEAR2 lacks the C-terminal EAR motif. ERF.F12-mEAR1 has a mutated EAR1 motif; ERF.F12-mEAR2 has a mutated EAR2 motif. D, ERF.F12 interacts with TPL2 and TPL4 through the C-terminal EAR motif in vitro. In an in vitro Co-IP assay, TPL2-FLAG and TPL4-FLAG bind to ERF.F12-HA and ERF.F12mEAR1-HA, but not ERF.F12mEAR2-HA. GFP-FLAG was used as a negative control.