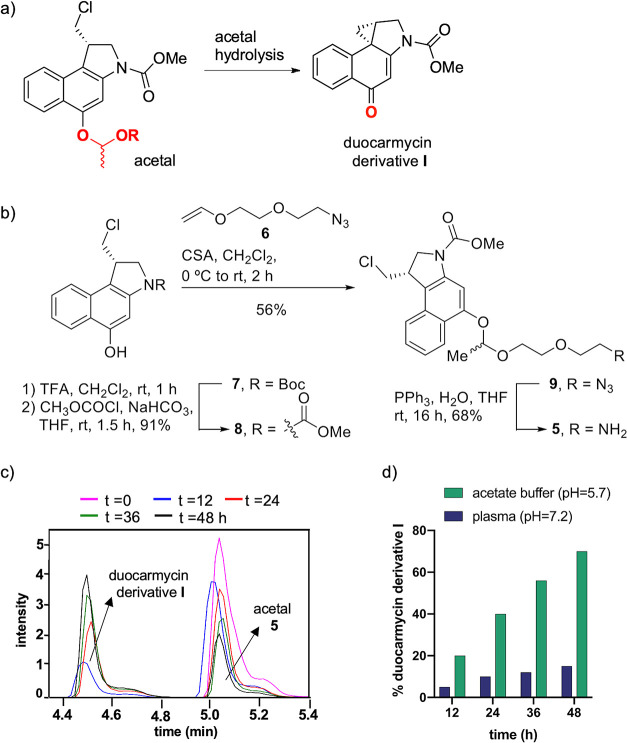
Figure 2.
(a) Formation of duocarmycin derivative I after hydrolysis of the acetal group. (b) Synthetic route to prepare acetal 5. (c) Monitoring of duocarmycin derivative I from acetal 5 at acidic pH [NaPi buffer (0.1 M, pH = 5.7)] and 37 °C by UPLC-MS. (d) Stability of acetal 5 at different pH values.