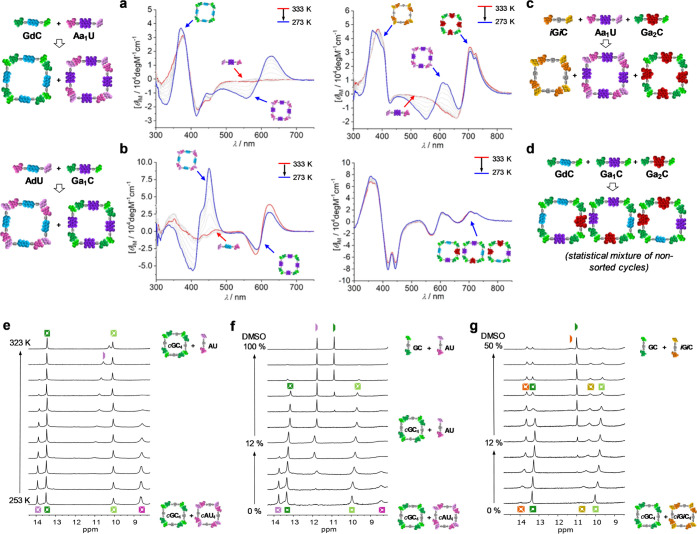
Figure 5.
Selective cyclic tetramer dissociation. (a–d) Temperature-dependent CD spectra in toluene of (a) a 1:1 GdC + Aa1U mixture, (b) a 1:1 AdU + Ga1C mixture, (c) a 1:1:1 iGiC + Aa1U + Ga2C mixture, and (d) a 1:1:1 GdC + Ga1C + Ga2C mixture. (e, f) Downfield region of the 1H NMR spectra of a 1:1 mixture of GC + AU in (e) CDCl3 with increasing temperature or (f) CDCl3:CCl4 (2:3) with increasing DMSO-D6 content. (g) Downfield region of the 1H NMR spectra of a 1:1 mixture of GC + iGiC in CDCl3 with increasing DMSO-D6 content. In the last mixture, the 1H signals of the c(iGiC)4 species are initially broad due to strong aggregation in pure CDCl3. A small amount of DMSO needs to be added to achieve complete solubility. For proton NMR codes, see Figure 1.